Strong Acids Weak Acids Calculations Strong Acids Recall




- Slides: 14

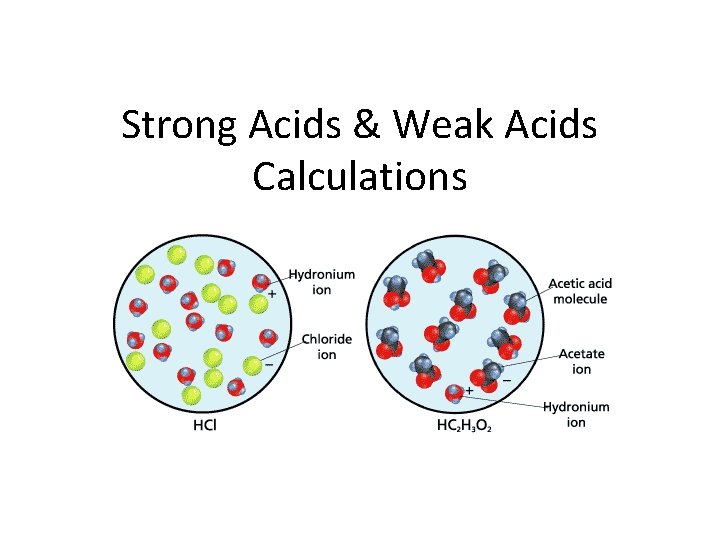
Strong Acids & Weak Acids Calculations

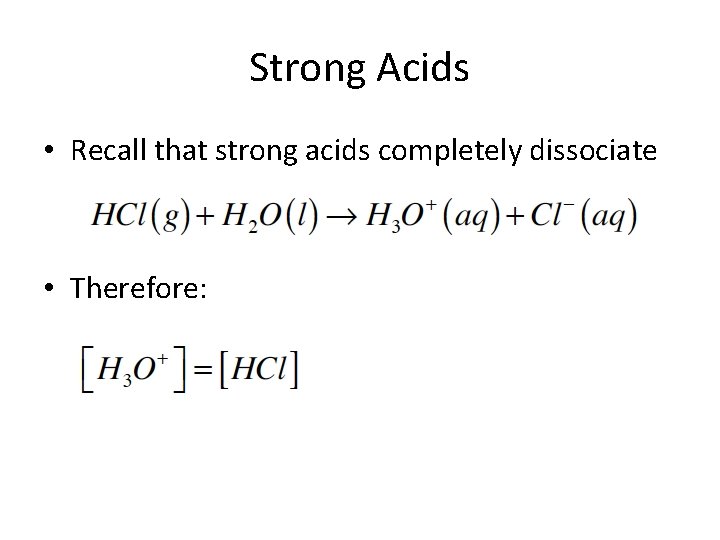
Strong Acids • Recall that strong acids completely dissociate • Therefore:

Examples of Strong Acids • • • There are relatively few strong acids: hydrochloric - HCl hydrobromic acid – HBr hydroiodic acid - HI sulphuric acid - H 2 SO 4 nitric acid – HNO 3 phosporic acid H 3 PO 4 perchloric acid – HCl. O 4 are the most familiar (these are the ones you are expected to know)

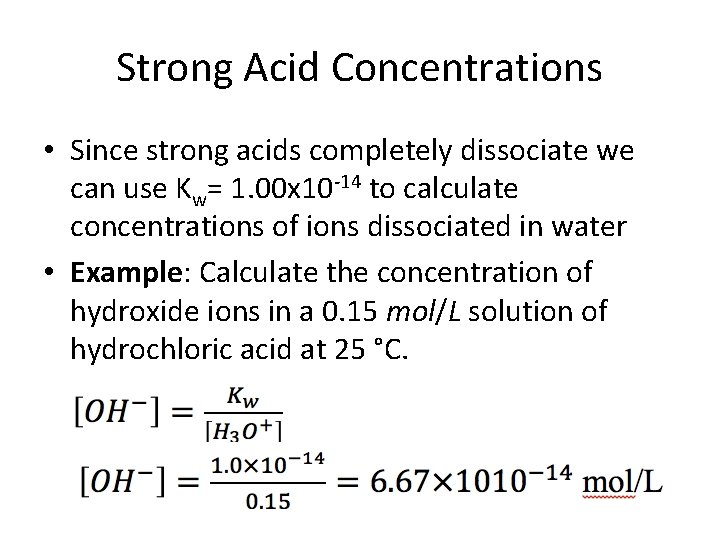
Strong Acid Concentrations • Since strong acids completely dissociate we can use Kw= 1. 00 x 10 -14 to calculate concentrations of ions dissociated in water • Example: Calculate the concentration of hydroxide ions in a 0. 15 mol/L solution of hydrochloric acid at 25 °C.

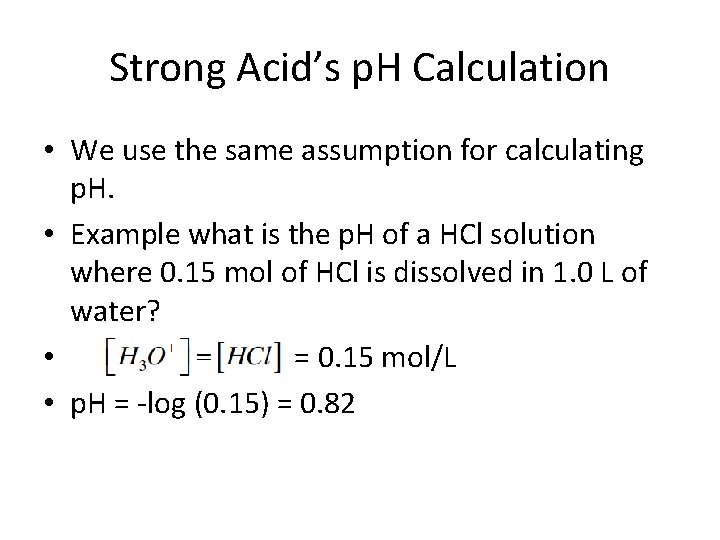
Strong Acid’s p. H Calculation • We use the same assumption for calculating p. H. • Example what is the p. H of a HCl solution where 0. 15 mol of HCl is dissolved in 1. 0 L of water? • = 0. 15 mol/L • p. H = -log (0. 15) = 0. 82

Strong Acid’s p. H Calculation • What is the p. OH of the same solution? • Recall p. H + p. OH = 14 • p. OH = 14 – 0. 83 = 13. 17 – We’ll just disregard sig figs for this one

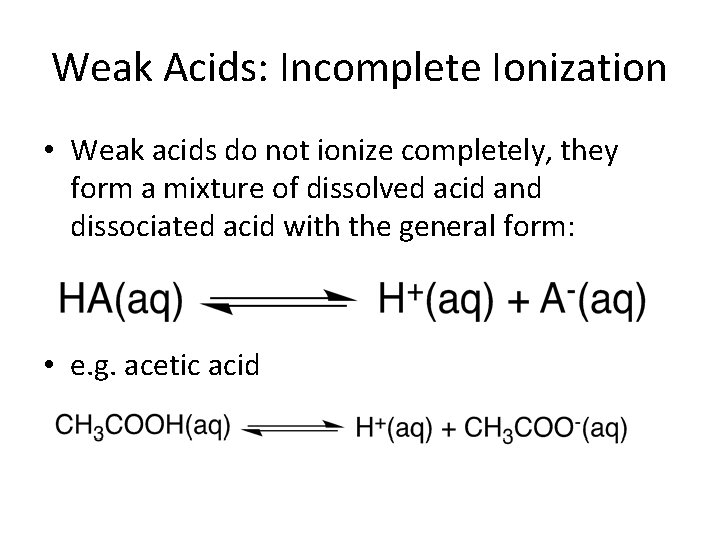
Weak Acids: Incomplete Ionization • Weak acids do not ionize completely, they form a mixture of dissolved acid and dissociated acid with the general form: • e. g. acetic acid

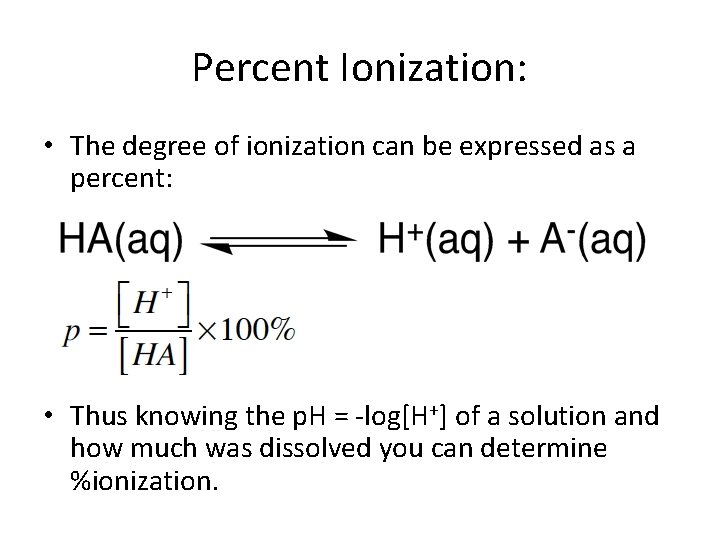
Percent Ionization: • The degree of ionization can be expressed as a percent: • Thus knowing the p. H = -log[H+] of a solution and how much was dissolved you can determine %ionization.

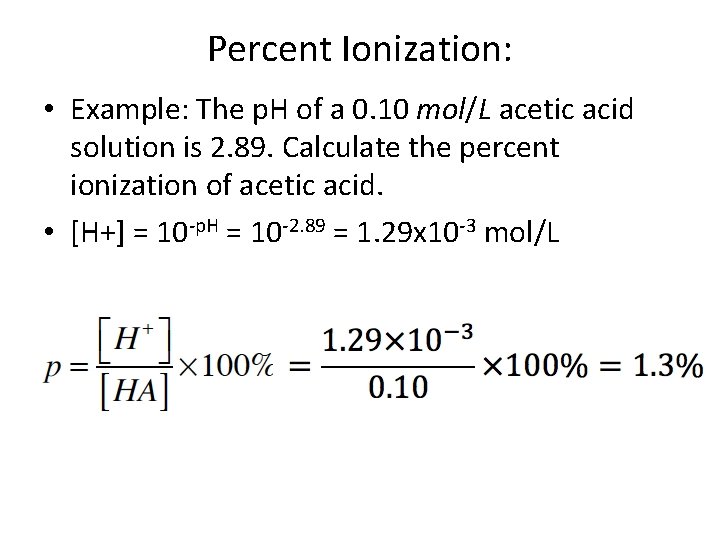
Percent Ionization: • Example: The p. H of a 0. 10 mol/L acetic acid solution is 2. 89. Calculate the percent ionization of acetic acid. • [H+] = 10 -p. H = 10 -2. 89 = 1. 29 x 10 -3 mol/L

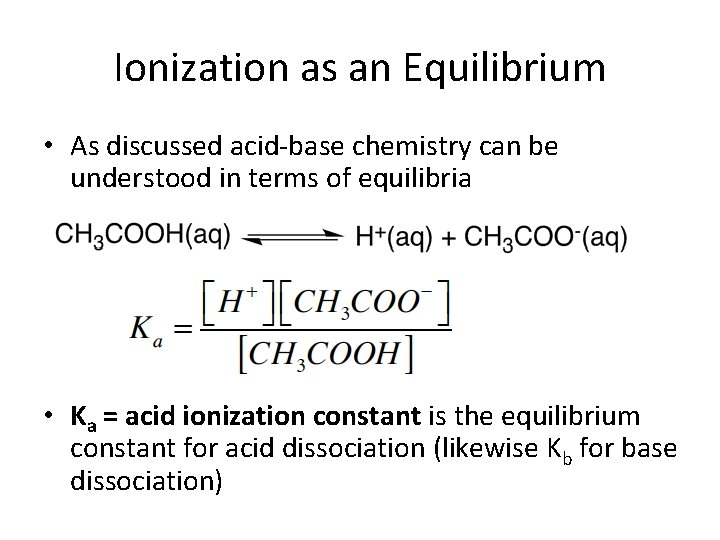
Ionization as an Equilibrium • As discussed acid-base chemistry can be understood in terms of equilibria • Ka = acid ionization constant is the equilibrium constant for acid dissociation (likewise Kb for base dissociation)

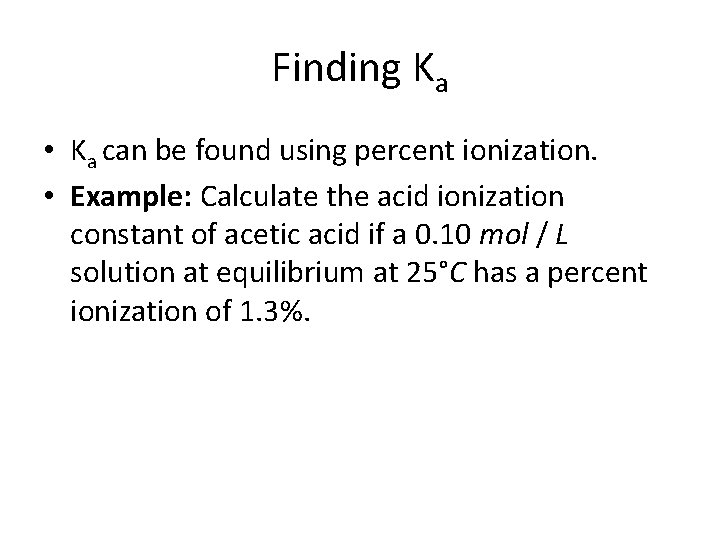
Finding Ka • Ka can be found using percent ionization. • Example: Calculate the acid ionization constant of acetic acid if a 0. 10 mol / L solution at equilibrium at 25°C has a percent ionization of 1. 3%.

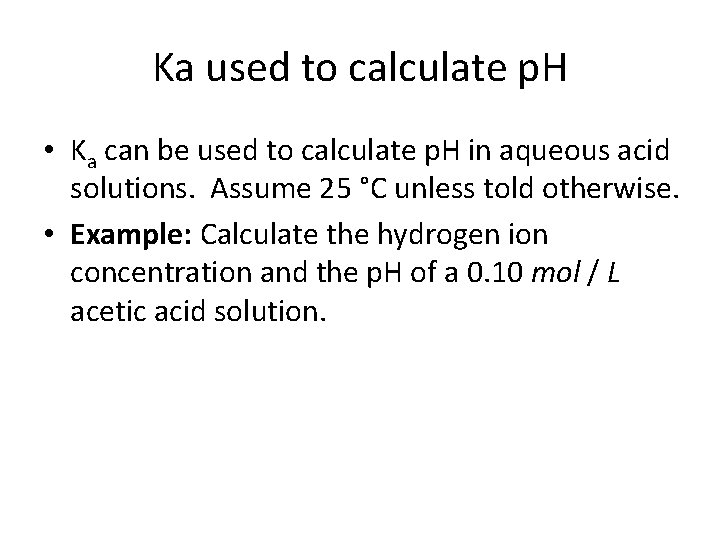
Ka used to calculate p. H • Ka can be used to calculate p. H in aqueous acid solutions. Assume 25 °C unless told otherwise. • Example: Calculate the hydrogen ion concentration and the p. H of a 0. 10 mol / L acetic acid solution.

p. H to Ka • Likewise p. H can be used to calculate Ka • Example: You measure the p. H of a 0. 10 mol/L hypochlorous acid (HOCl ) solution and find it to be 4. 23. What is the Ka for hypochlorous acid?

Likewise for Bases • All of these calculations are applicable to strong bases and weak bases but we will not be covering them.