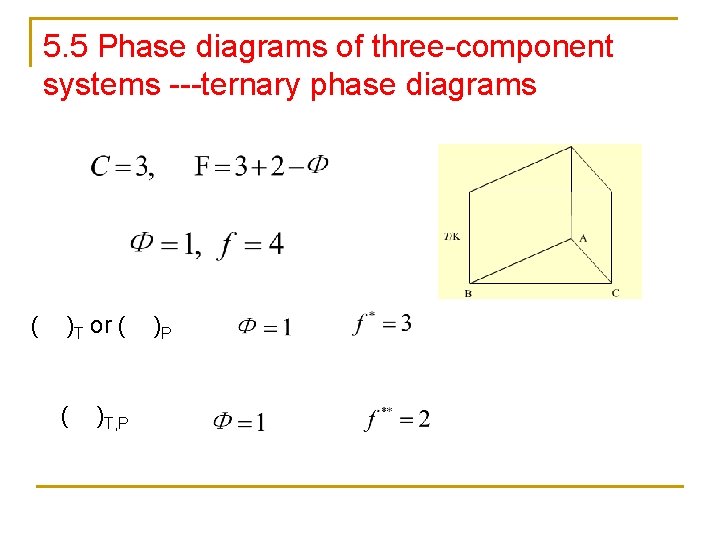
5 5 Phase diagrams of threecomponent systems ternary

- Slides: 30

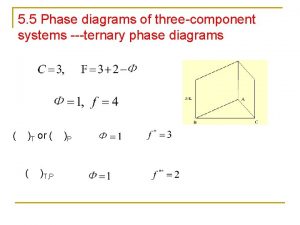
5. 5 Phase diagrams of three-component systems ---ternary phase diagrams ( )T or ( ( )T, P )P

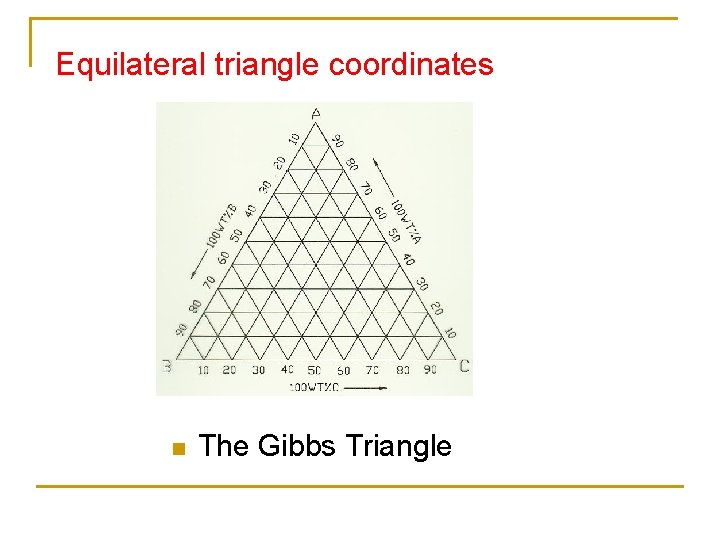
Equilateral triangle coordinates n The Gibbs Triangle

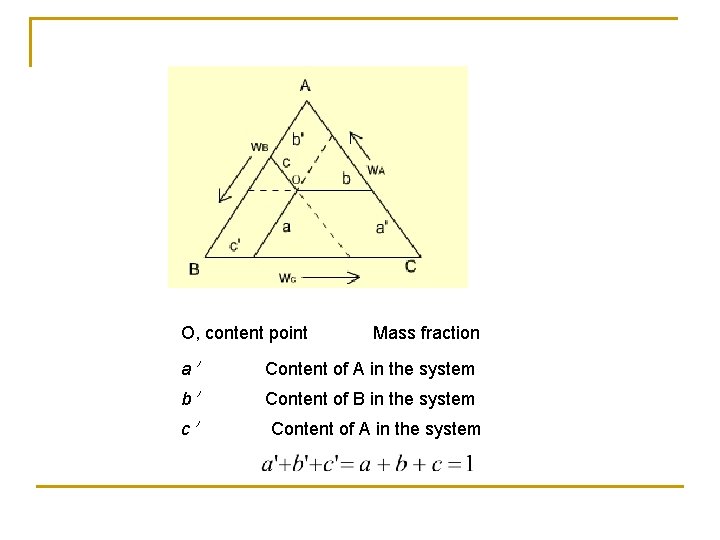
O, content point Mass fraction a' Content of A in the system b' Content of B in the system c' Content of A in the system

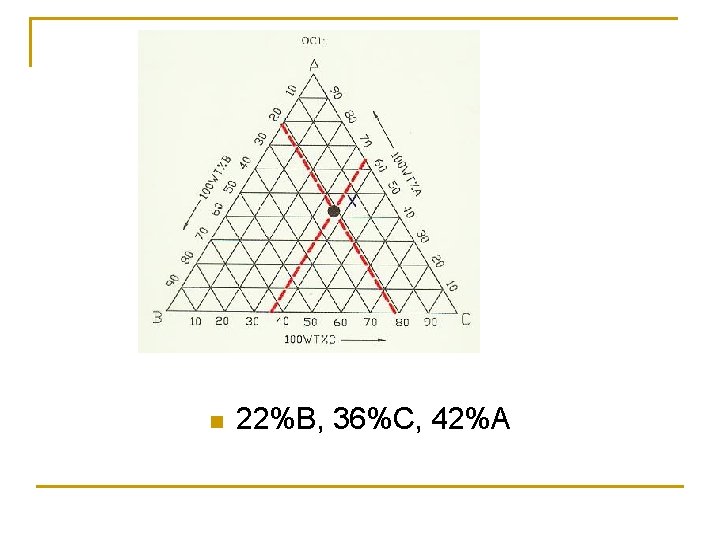
n 22%B, 36%C, 42%A

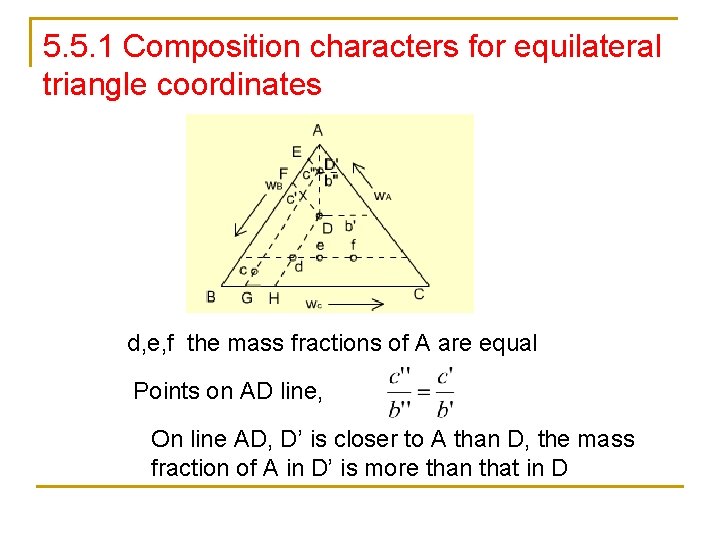
5. 5. 1 Composition characters for equilateral triangle coordinates d, e, f the mass fractions of A are equal Points on AD line, On line AD, D’ is closer to A than D, the mass fraction of A in D’ is more than that in D

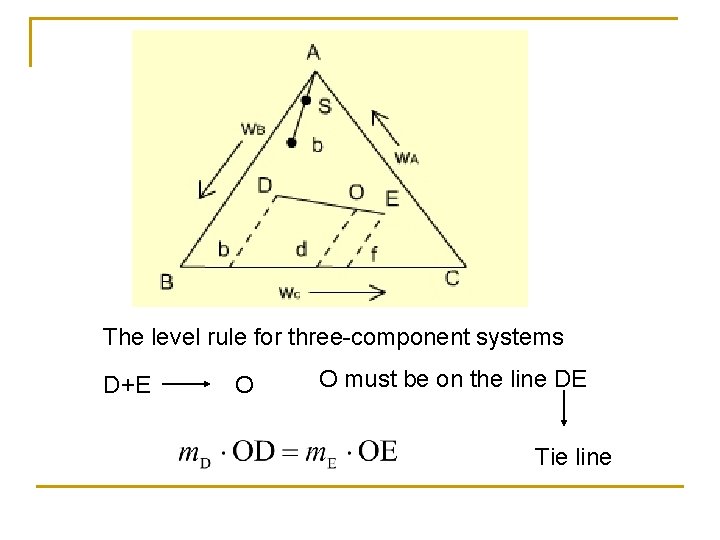
The level rule for three-component systems D+E O O must be on the line DE Tie line

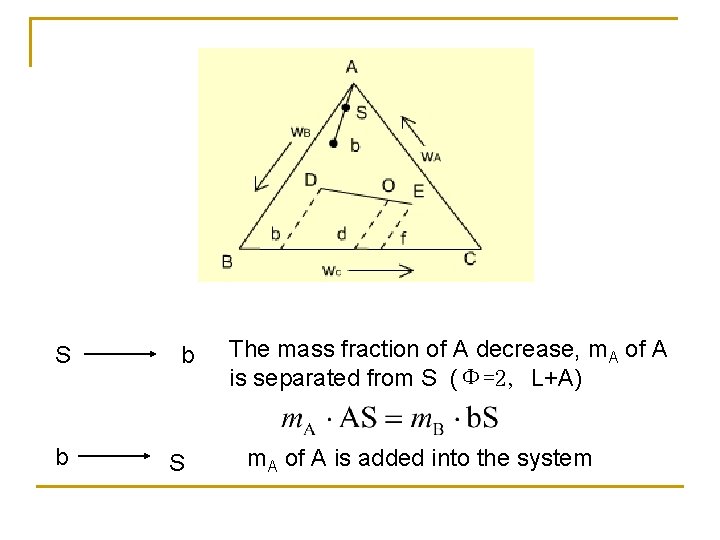
S b b S The mass fraction of A decrease, m. A of A is separated from S (Φ=2, L+A) m. A of A is added into the system

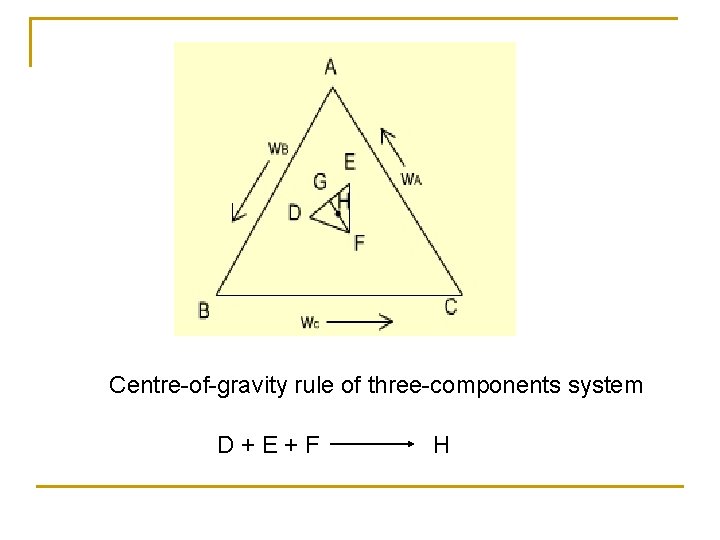
Centre-of-gravity rule of three-components system D+E+F H

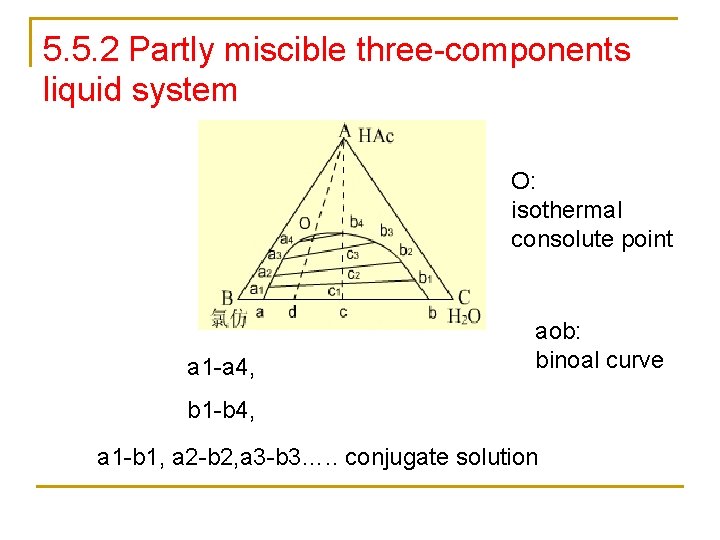
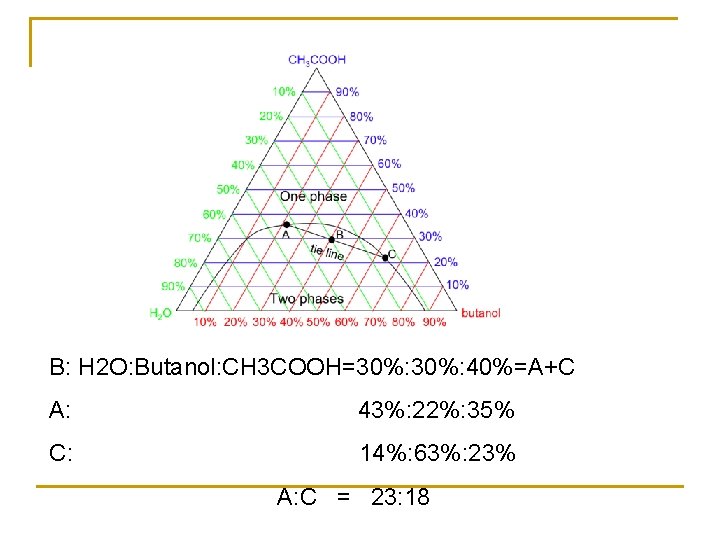
5. 5. 2 Partly miscible three-components liquid system O: isothermal consolute point a 1 -a 4, aob: binoal curve b 1 -b 4, a 1 -b 1, a 2 -b 2, a 3 -b 3…. . conjugate solution

B: H 2 O: Butanol: CH 3 COOH=30%: 40%=A+C A: 43%: 22%: 35% C: 14%: 63%: 23% A: C = 23: 18

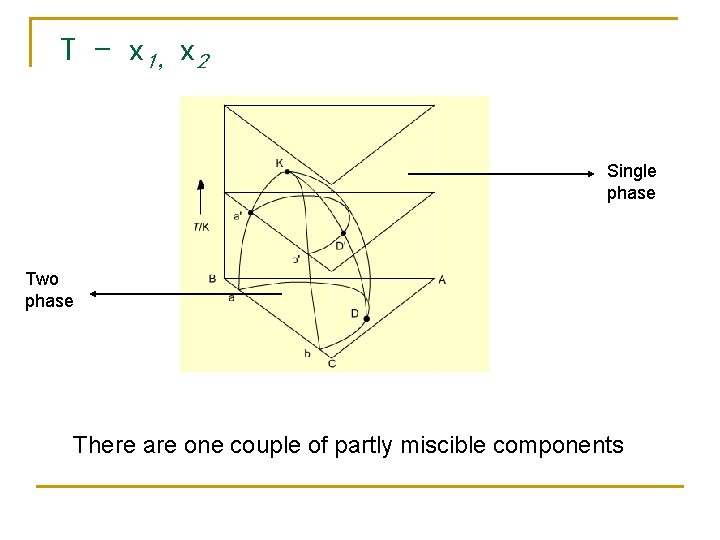
T - x 1,x 2 Single phase Two phase There are one couple of partly miscible components

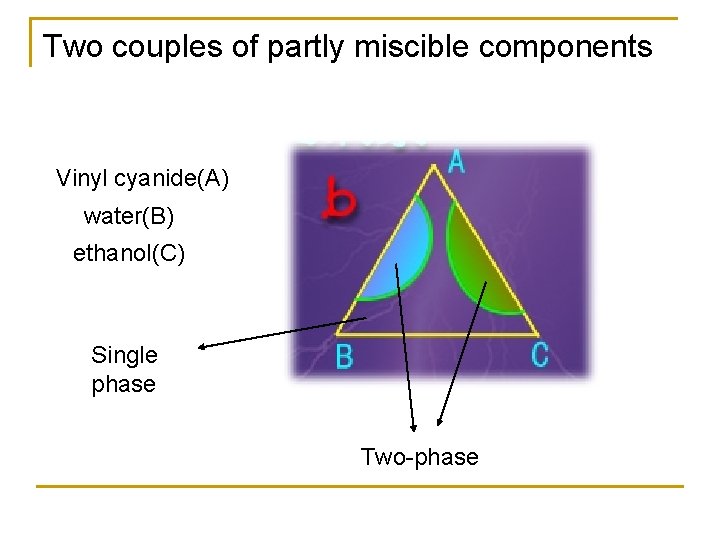
Two couples of partly miscible components Vinyl cyanide(A) water(B) ethanol(C) Single phase Two-phase

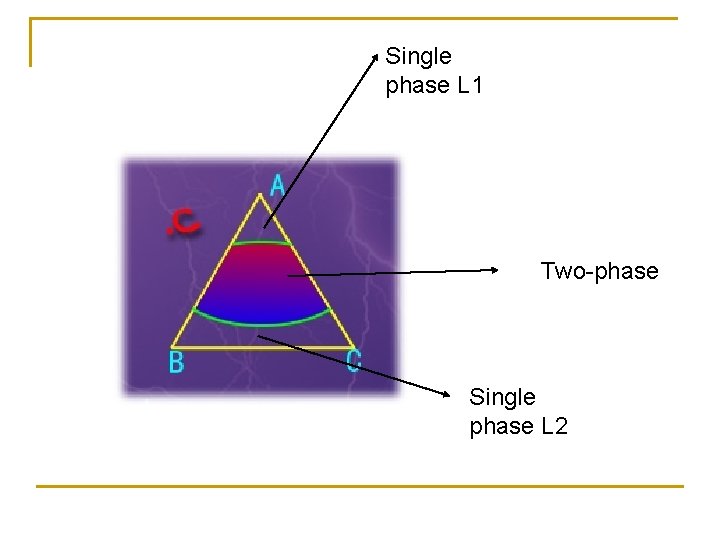
Single phase L 1 Two-phase Single phase L 2

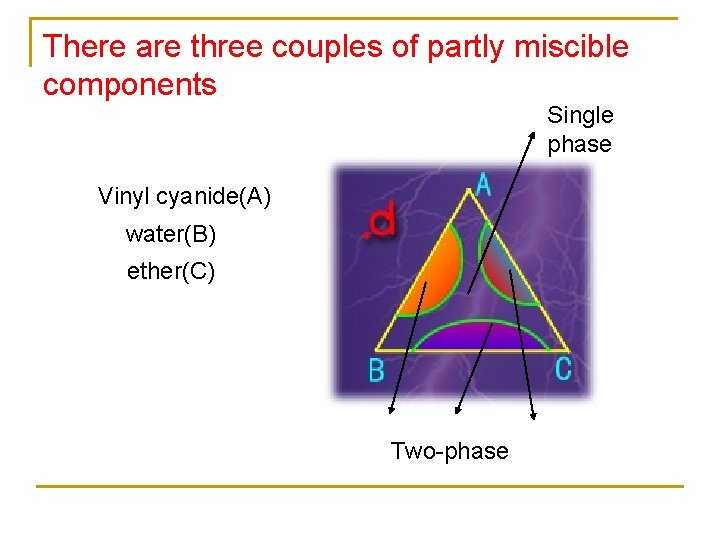
There are three couples of partly miscible components Single phase Vinyl cyanide(A) water(B) ether(C) Two-phase

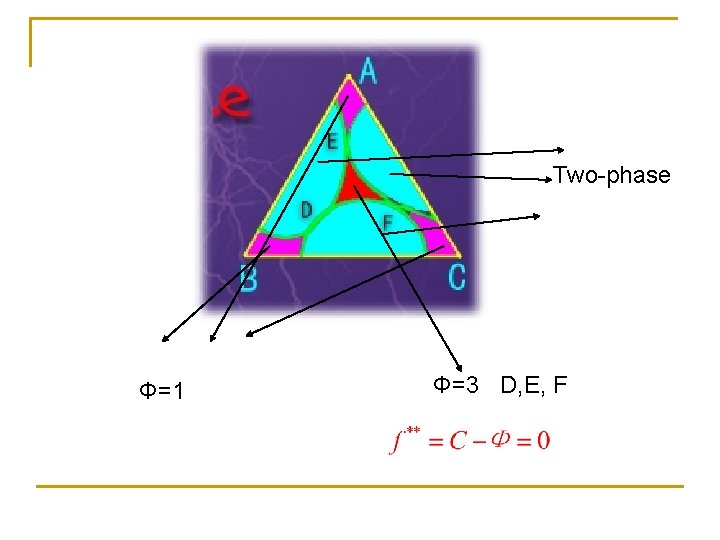
Two-phase Φ=1 Φ=3 D, E, F

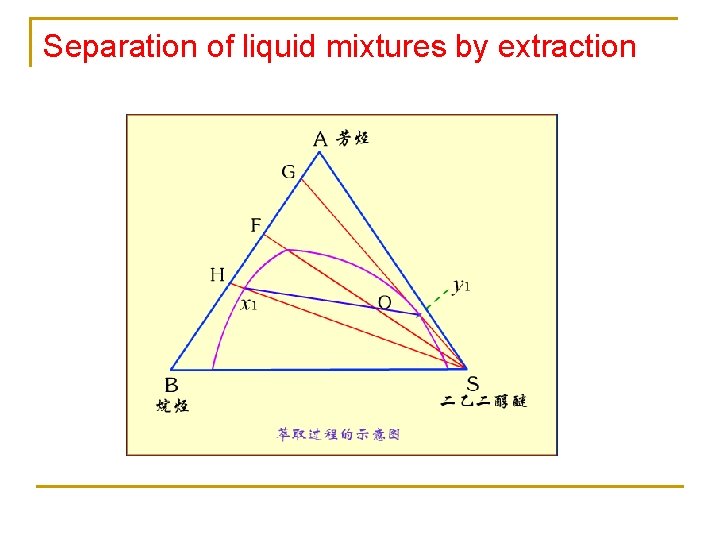
Separation of liquid mixtures by extraction

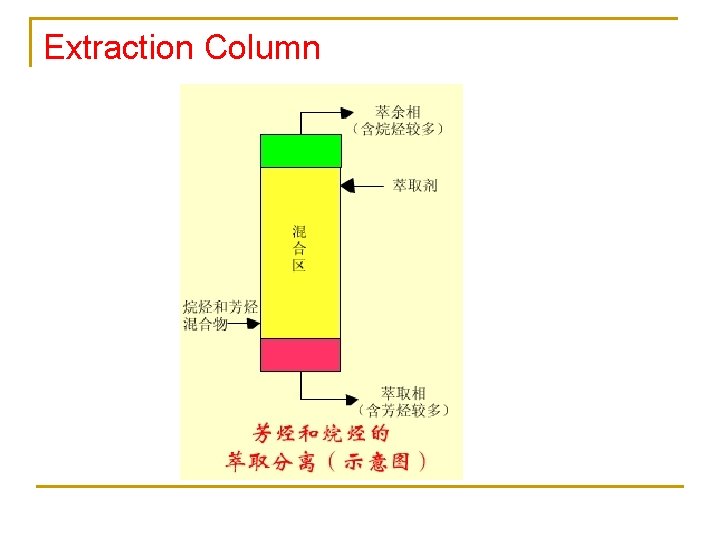
Extraction Column

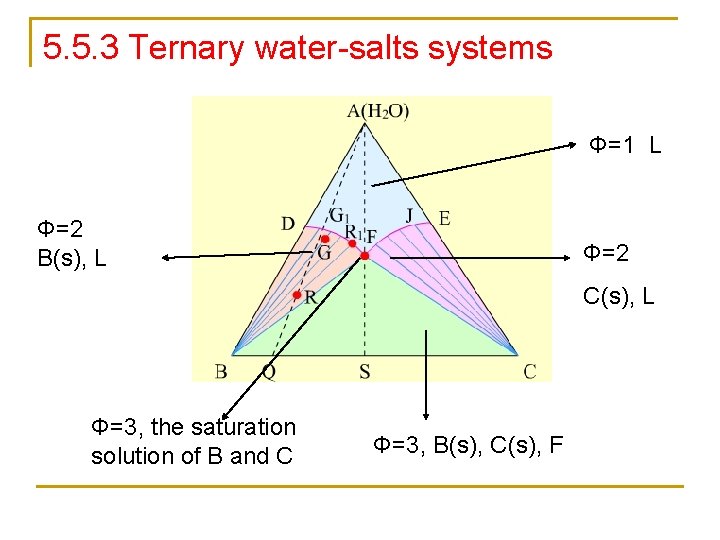
5. 5. 3 Ternary water-salts systems Φ=1 L Φ=2 B(s), L Φ=2 C(s), L Φ=3, the saturation solution of B and C Φ=3, B(s), C(s), F

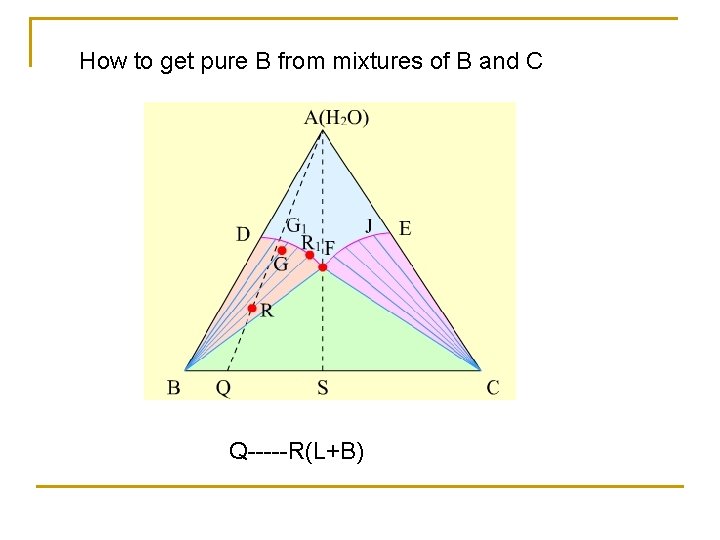
How to get pure B from mixtures of B and C Q-----R(L+B)

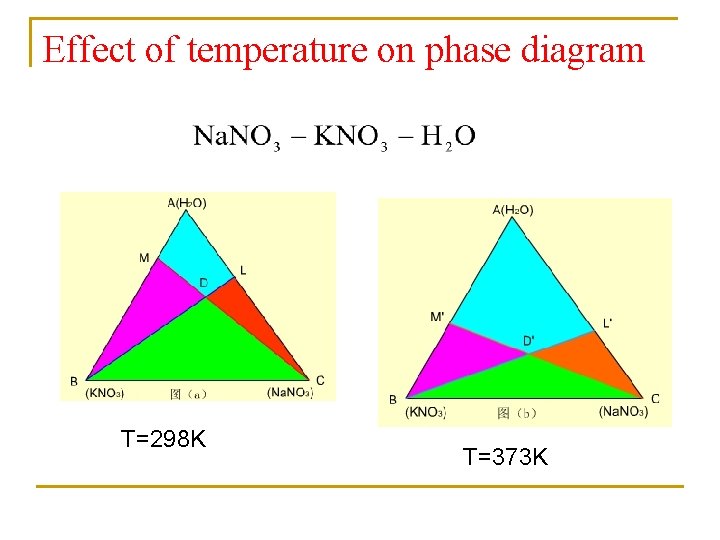
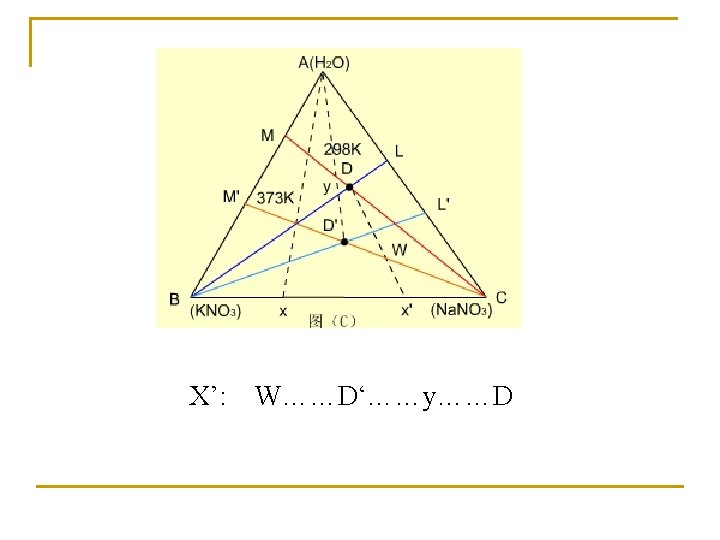
Effect of temperature on phase diagram T=298 K T=373 K

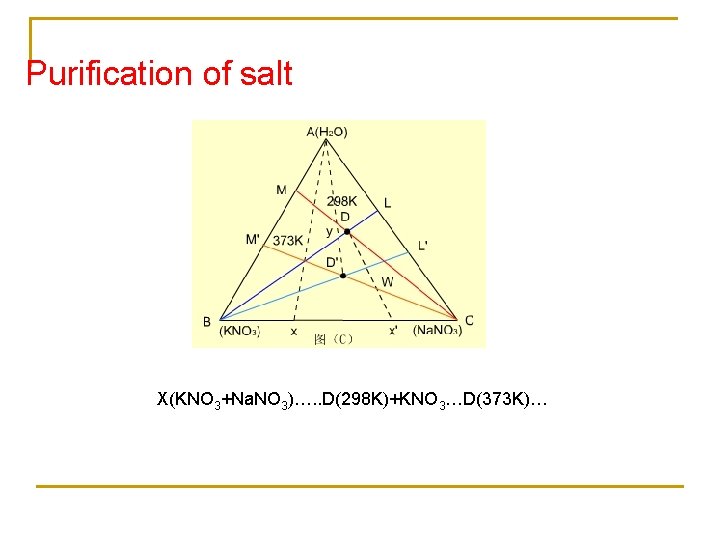
Purification of salt X(KNO 3+Na. NO 3)…. . D(298 K)+KNO 3…D(373 K)…


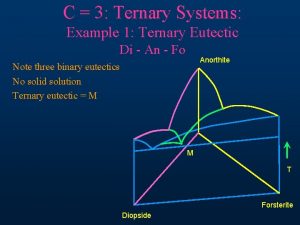
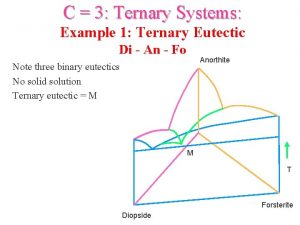
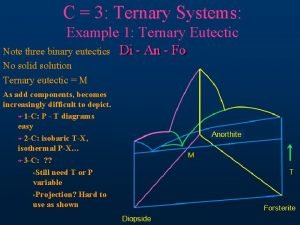
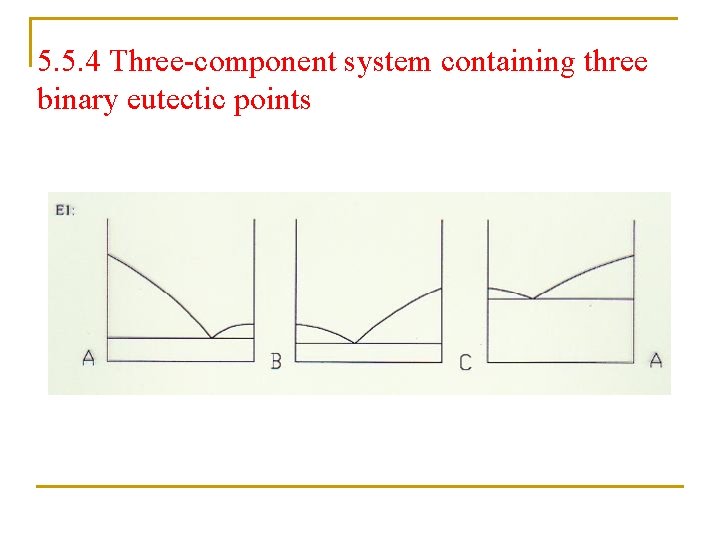
5. 5. 4 Three-component system containing three binary eutectic points

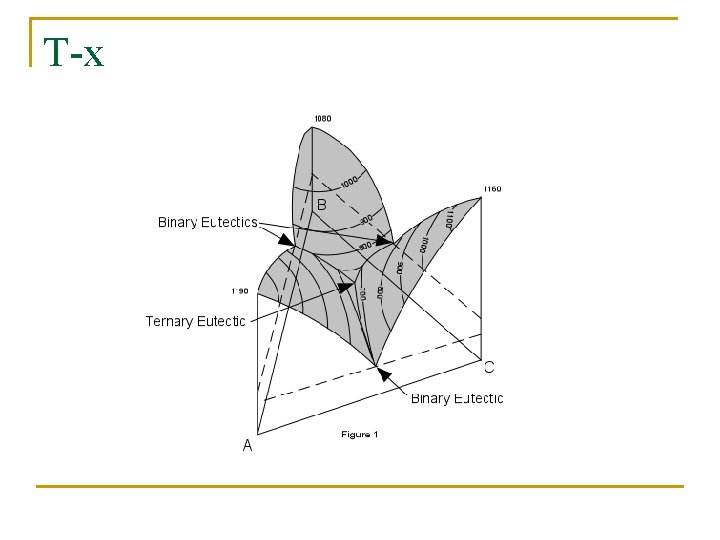
T-x

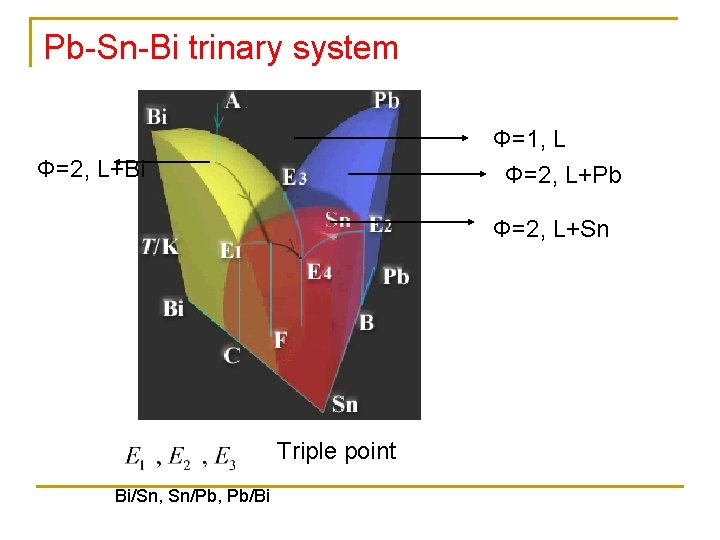
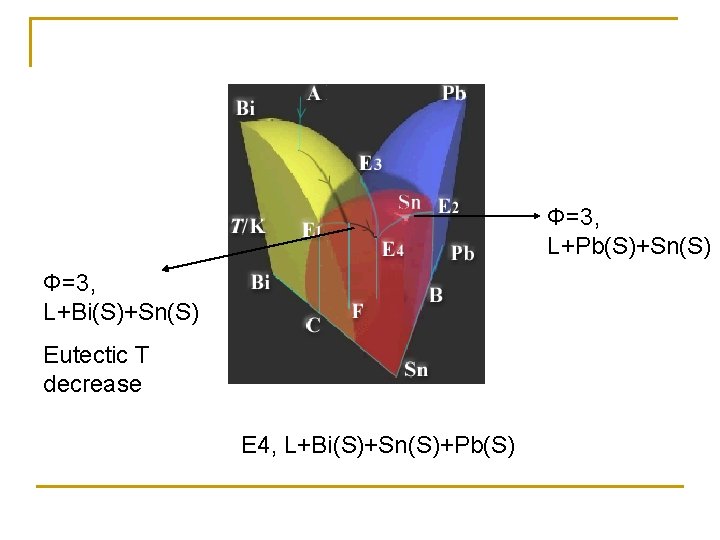
Pb-Sn-Bi trinary system Φ=1, L Φ=2, L+Bi Φ=2, L+Pb Φ=2, L+Sn Triple point Bi/Sn, Sn/Pb, Pb/Bi

Φ=3, L+Pb(S)+Sn(S) Φ=3, L+Bi(S)+Sn(S) Eutectic T decrease E 4, L+Bi(S)+Sn(S)+Pb(S)

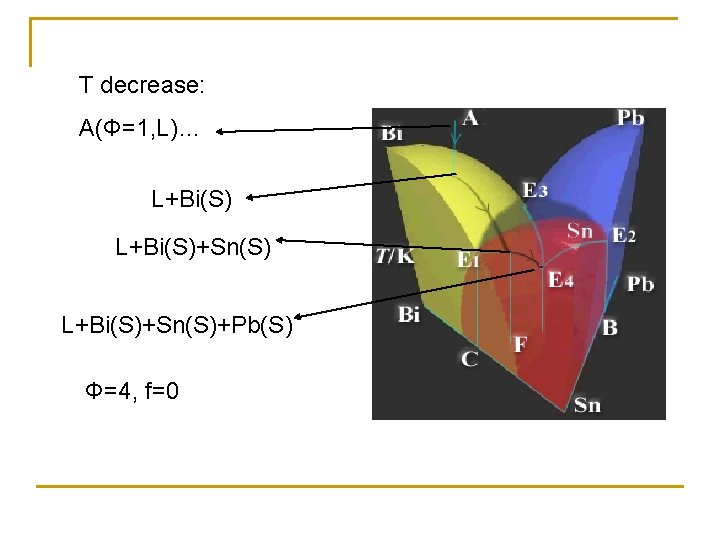
T decrease: A(Φ=1, L)… L+Bi(S)+Sn(S)+Pb(S) Φ=4, f=0

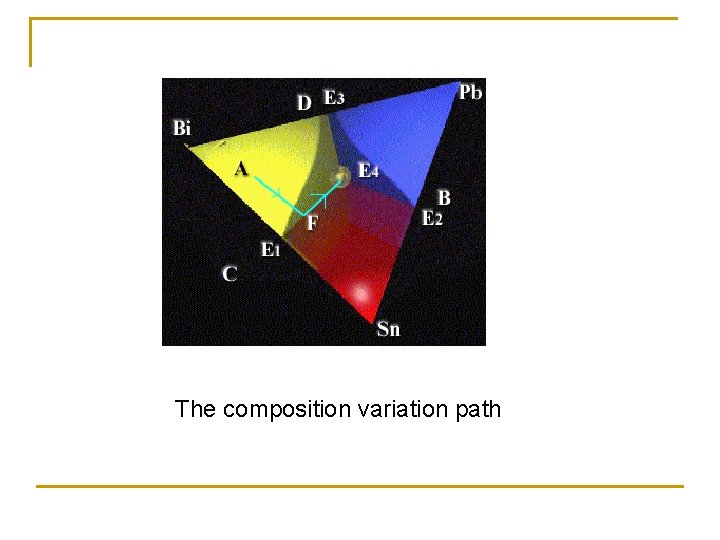
The composition variation path

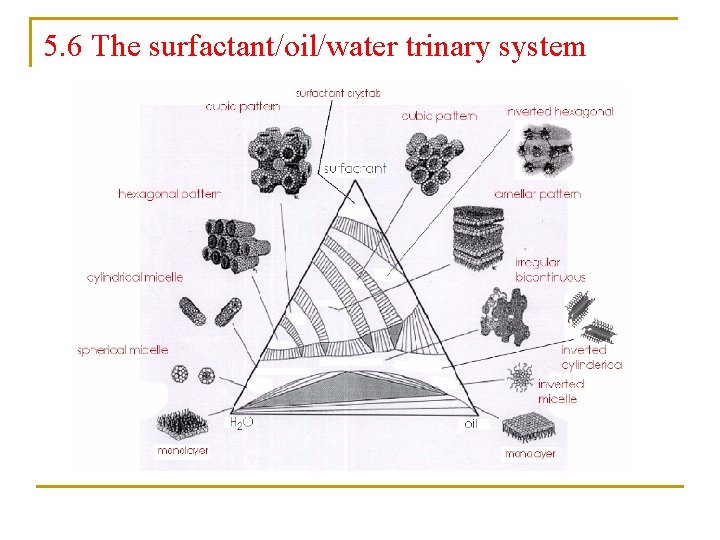
5. 6 The surfactant/oil/water trinary system

Homework n Y: P 188 39 n Review the whole chapter
 Di an fo ternary phase diagram
Di an fo ternary phase diagram Igneous ternary diagram
Igneous ternary diagram The interaction diagrams, use case diagrams are called as
The interaction diagrams, use case diagrams are called as Uml activity diagram tutorial
Uml activity diagram tutorial Interpreting phase diagrams
Interpreting phase diagrams Unlabeled phase diagram
Unlabeled phase diagram Interpreting phase diagrams
Interpreting phase diagrams Phase diagrams
Phase diagrams Unary and binary phase diagram
Unary and binary phase diagram Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography M tswett pronunciation
M tswett pronunciation Mobile phase and stationary phase
Mobile phase and stationary phase Mobile phase vs stationary phase
Mobile phase vs stationary phase Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Line vs phase voltage
Line vs phase voltage Which detector used in hplc
Which detector used in hplc In a triangle connected source feeding a y connected load
In a triangle connected source feeding a y connected load Broad phase vs narrow phase
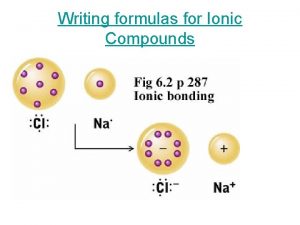
Broad phase vs narrow phase Ternary ionic compounds
Ternary ionic compounds Cl
Cl Weak entity database
Weak entity database A sorting technique is called stable if: *
A sorting technique is called stable if: * How tcam works
How tcam works Pyroxene ternary diagram
Pyroxene ternary diagram Ternary mixture separation
Ternary mixture separation Conditional operator in c
Conditional operator in c Ternary ionic compounds
Ternary ionic compounds Ternary heap
Ternary heap Ternary relationship sql
Ternary relationship sql Erdplus ternary relationship
Erdplus ternary relationship Ternary ionic compounds
Ternary ionic compounds