Lecture 15 Phase Diagrams Dr A K M



- Slides: 29

Lecture -15 Phase Diagrams Dr. A. K. M. SHAFIQUL ISLAM Dept. of Chemical Engineering Technology Industrial Biotechnology 28/05/2015 2/26/2021 1

Subtopics �Definitions �The Phase Rule �Two-component Systems: ü Vapour pressure Diagrams ü Temperature-composition Diagrams ü Liquid-liquid Phase Diagrams ü Liquid-solid Phase Diagrams 2/26/2021 2

Definitions �A phase of a substance is a form of matter that is uniform throughout in chemical composition & physical state. �Example �Solid, liquid and gas phase of substance. �The black and white allotropes of phosphate. �The aragonite and calcite polymorphs of calcium carbonate. 2/26/2021 3

Definitions �The number of phase is denoted by P. �A gas or gas mixture is a single phase (P = 1). �A crystal of substance is single phase. �Two fully miscible liquid form a single phase 2/26/2021 4

Definitions �A solution of Na. Cl in water is a single phase (P=1). �Ice is a single phase. Ø A slurry of ice & water is two-phase system (P=2). Ø A calcium carbonate system undergoes thermal deposition – two solid phase (Ca. CO 3 & Ca. O) one gaseous phase (CO 2) (P=3) Ø An alloy of two metals is a two-phase system (P=2) if they are immiscible Ø but a single-phase system (P=1) if they are miscible. 2/26/2021 5

Definitions �A dispersion is uniform on a macroscopic scale but not a microscopic scale, for it consists of grains or droplets of one substance in a matrix of the other. �A small sample could come entirely from one of the minute grain A and would not be representive of the whole. �Constituent – a chemical species (an ion or a molecule) that is present. Ø A mixture of ethanol & water – Two constituents. Ø A solution of sodium chloride – Three constituents (water, Na + ions & Cl- ions). �Component – a chemically independent constituent of a system. 2/26/2021 6

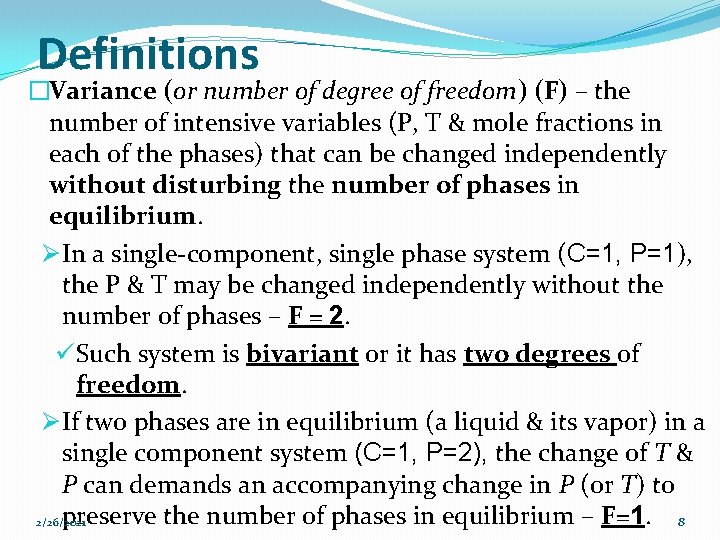
Example 1 �There are Three phases & Three constituents. �To specify the composition of Phase 3 – need CO 2 Ø To specify the composition of Phase 2 – need Ca. O Ø To specify the composition of Phase 1 – do not need an additional species (stoichiometry). �The system has only Two components (C=2). 2/26/2021 7

Definitions �Variance (or number of degree of freedom) (F) – the number of intensive variables (P, T & mole fractions in each of the phases) that can be changed independently without disturbing the number of phases in equilibrium. ØIn a single-component, single phase system (C=1, P=1), the P & T may be changed independently without the number of phases – F = 2. ü Such system is bivariant or it has two degrees of freedom. ØIf two phases are in equilibrium (a liquid & its vapor) in a single component system (C=1, P=2), the change of T & P can demands an accompanying change in P (or T) to preserve the number of phases in equilibrium – F=1. 8 2/26/2021

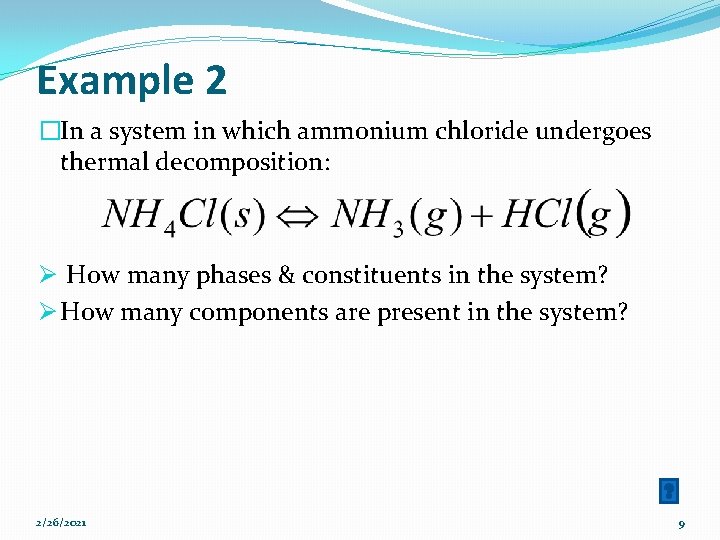
Example 2 �In a system in which ammonium chloride undergoes thermal decomposition: Ø How many phases & constituents in the system? Ø How many components are present in the system? 2/26/2021 9

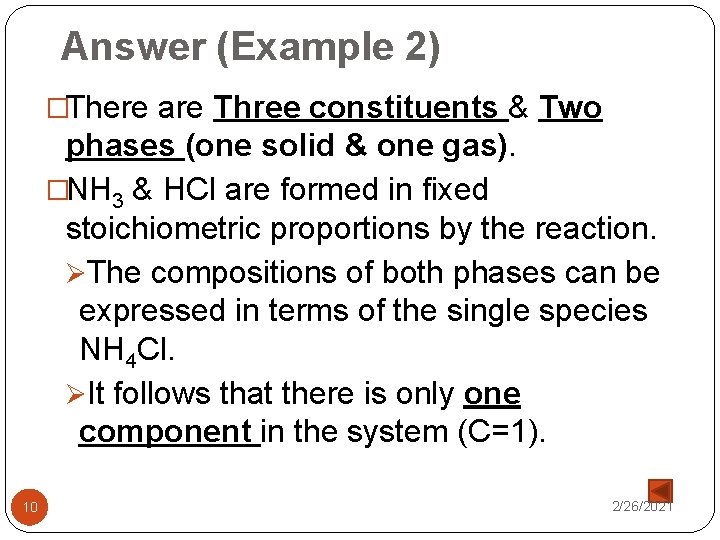
Answer (Example 2) �There are Three constituents & Two phases (one solid & one gas). �NH 3 & HCl are formed in fixed stoichiometric proportions by the reaction. ØThe compositions of both phases can be expressed in terms of the single species NH 4 Cl. ØIt follows that there is only one component in the system (C=1). 10 2/26/2021

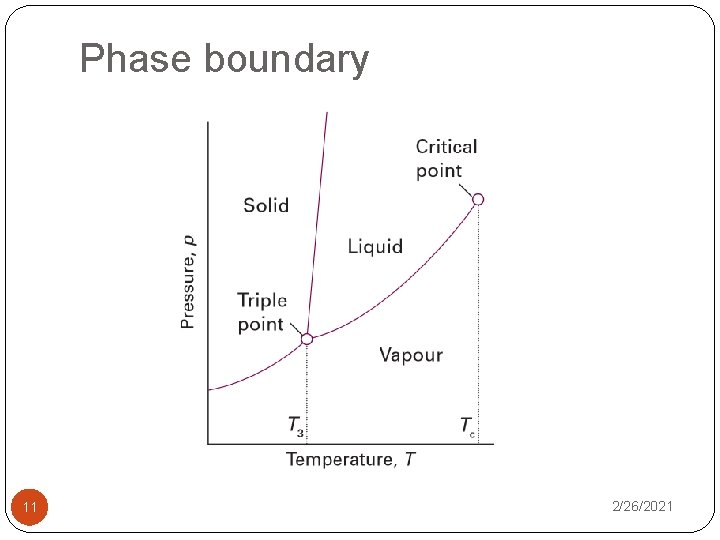
Phase boundary 11 2/26/2021

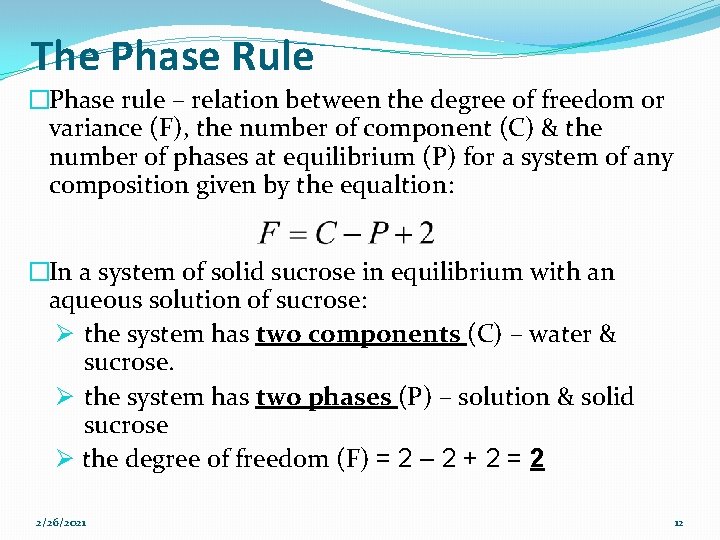
The Phase Rule �Phase rule – relation between the degree of freedom or variance (F), the number of component (C) & the number of phases at equilibrium (P) for a system of any composition given by the equaltion: �In a system of solid sucrose in equilibrium with an aqueous solution of sucrose: Ø the system has two components (C) – water & sucrose. Ø the system has two phases (P) – solution & solid sucrose Ø the degree of freedom (F) = 2 – 2 + 2 = 2 2/26/2021 12

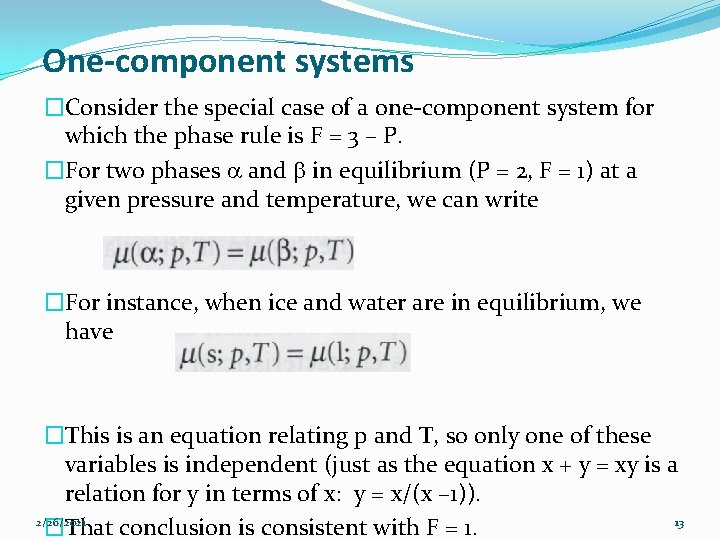
One-component systems �Consider the special case of a one-component system for which the phase rule is F = 3 – P. �For two phases a and b in equilibrium (P = 2, F = 1) at a given pressure and temperature, we can write �For instance, when ice and water are in equilibrium, we have �This is an equation relating p and T, so only one of these variables is independent (just as the equation x + y = xy is a relation for y in terms of x: y = x/(x – 1)). 2/26/2021 13 �That conclusion is consistent with F = 1.

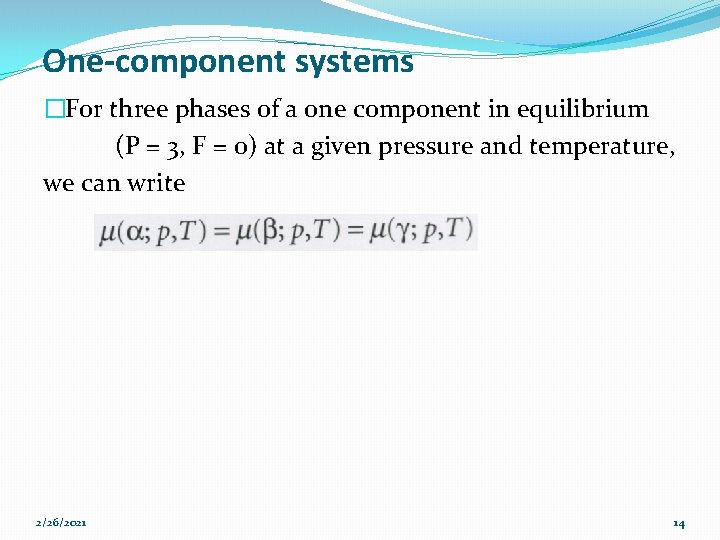
One-component systems �For three phases of a one component in equilibrium (P = 3, F = 0) at a given pressure and temperature, we can write 2/26/2021 14

Two-component systems �When two components are present in a system: Ø C = 2, F=4 -P. Ø If the temperature is constant, F = 3 -P (which max value of 2). Ø One of these two remaining F – P & composition (mole fraction of one component). Ø One form of the phase diagram is a map of P & compositions at which each phase is stable. �Alternatively, the pressure could be held constant & the phase diagram depicted in terms of T & composition. 2/26/2021 15

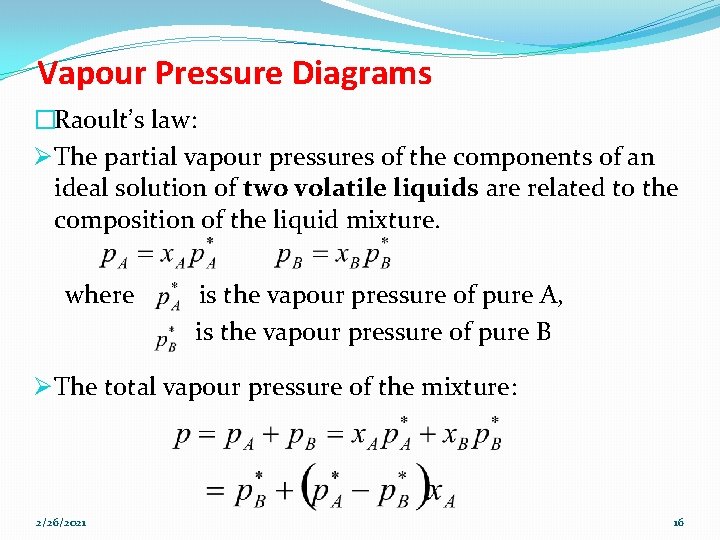
Vapour Pressure Diagrams �Raoult’s law: Ø The partial vapour pressures of the components of an ideal solution of two volatile liquids are related to the composition of the liquid mixture. where is the vapour pressure of pure A, is the vapour pressure of pure B Ø The total vapour pressure of the mixture: 2/26/2021 16

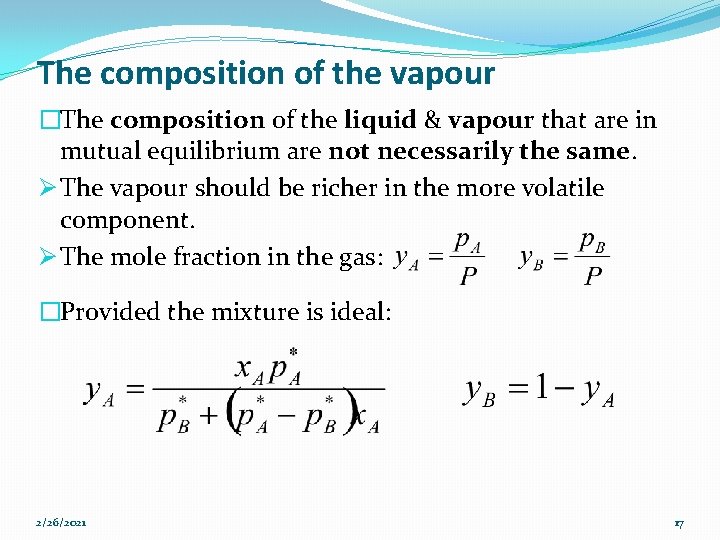
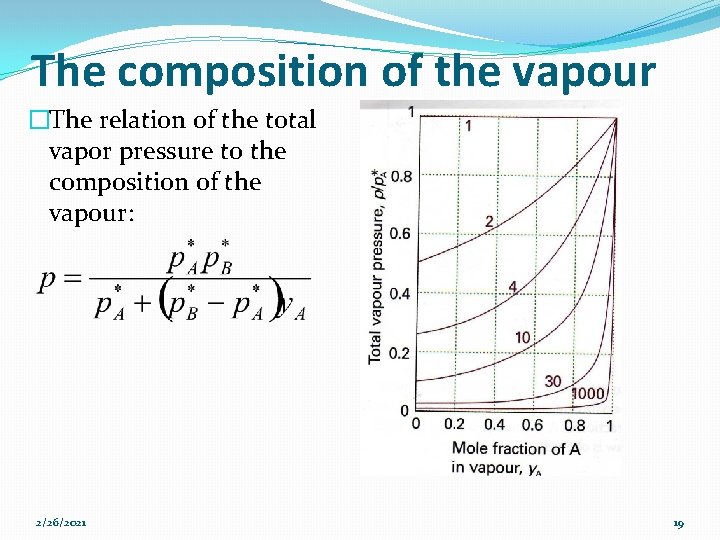
The composition of the vapour �The composition of the liquid & vapour that are in mutual equilibrium are not necessarily the same. Ø The vapour should be richer in the more volatile component. Ø The mole fraction in the gas: �Provided the mixture is ideal: 2/26/2021 17

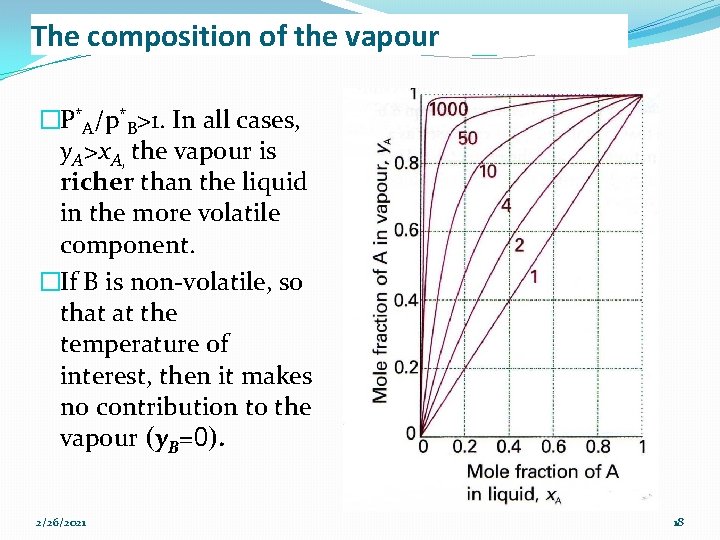
The composition of the vapour �P*A/p*B>1. In all cases, y. A>x. A, the vapour is richer than the liquid in the more volatile component. �If B is non-volatile, so that at the temperature of interest, then it makes no contribution to the vapour (y. B=0). 2/26/2021 18

The composition of the vapour �The relation of the total vapor pressure to the composition of the vapour: 2/26/2021 19

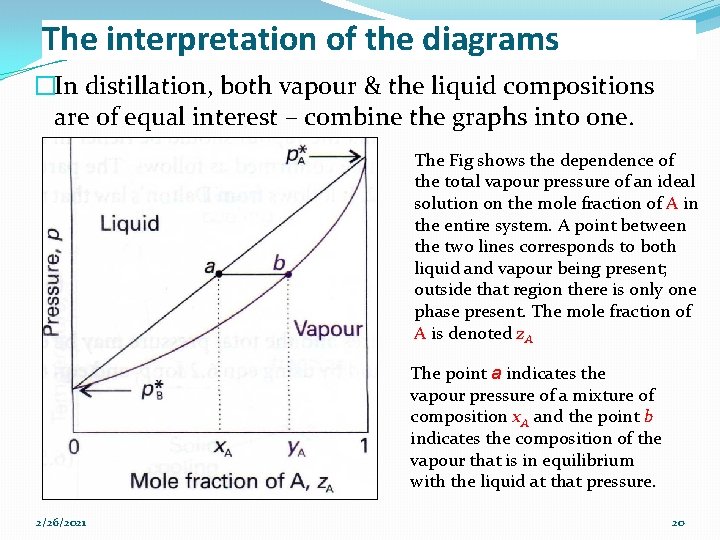
The interpretation of the diagrams �In distillation, both vapour & the liquid compositions are of equal interest – combine the graphs into one. The Fig shows the dependence of the total vapour pressure of an ideal solution on the mole fraction of A in the entire system. A point between the two lines corresponds to both liquid and vapour being present; outside that region there is only one phase present. The mole fraction of A is denoted z. A The point a indicates the vapour pressure of a mixture of composition x. A and the point b indicates the composition of the vapour that is in equilibrium with the liquid at that pressure. 2/26/2021 20

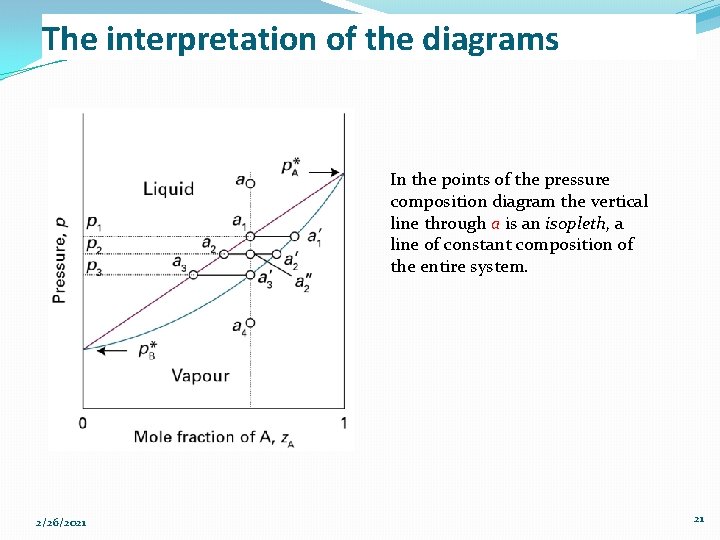
The interpretation of the diagrams In the points of the pressure composition diagram the vertical line through a is an isopleth, a line of constant composition of the entire system. 2/26/2021 21

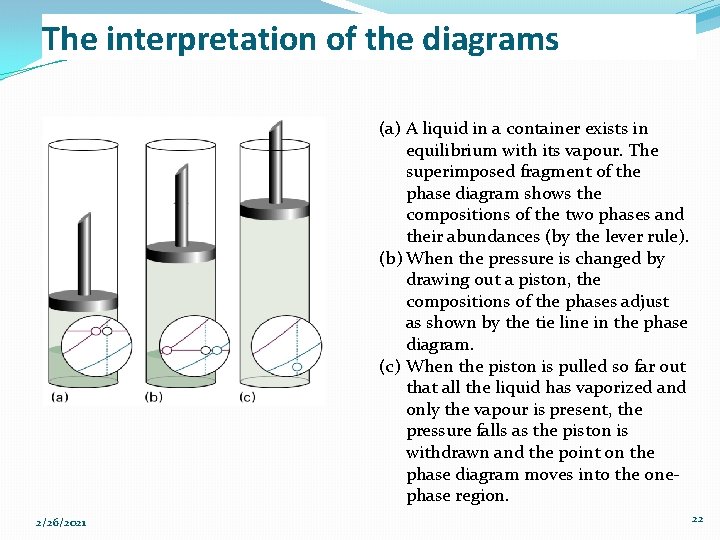
The interpretation of the diagrams (a) A liquid in a container exists in equilibrium with its vapour. The superimposed fragment of the phase diagram shows the compositions of the two phases and their abundances (by the lever rule). (b) When the pressure is changed by drawing out a piston, the compositions of the phases adjust as shown by the tie line in the phase diagram. (c) When the piston is pulled so far out that all the liquid has vaporized and only the vapour is present, the pressure falls as the piston is withdrawn and the point on the phase diagram moves into the onephase region. 2/26/2021 22

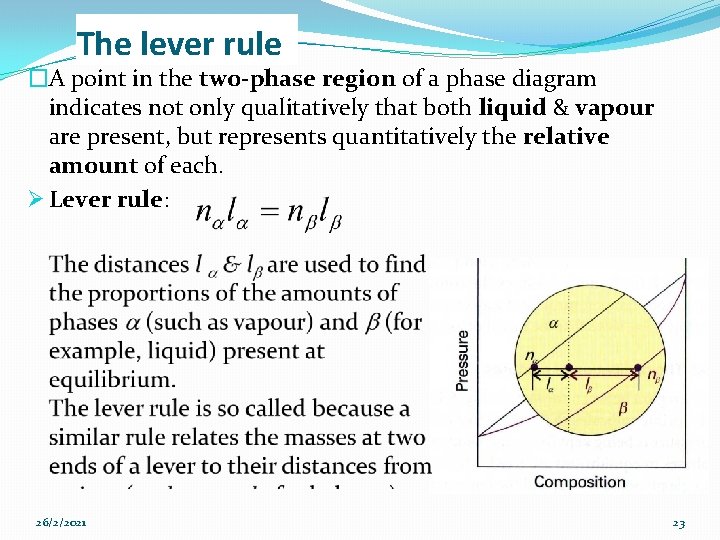
The lever rule �A point in the two-phase region of a phase diagram indicates not only qualitatively that both liquid & vapour are present, but represents quantitatively the relative amount of each. Ø Lever rule: 26/2/2021 23

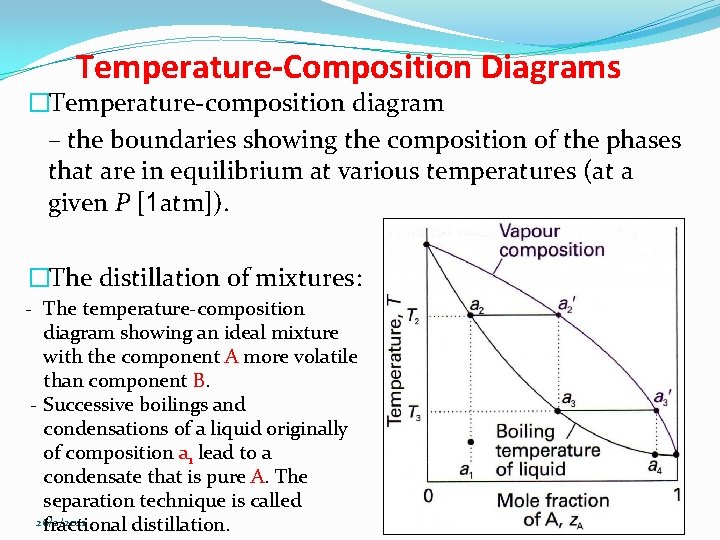
Temperature-Composition Diagrams �Temperature-composition diagram – the boundaries showing the composition of the phases that are in equilibrium at various temperatures (at a given P [1 atm]). �The distillation of mixtures: - The temperature-composition diagram showing an ideal mixture with the component A more volatile than component B. - Successive boilings and condensations of a liquid originally of composition a 1 lead to a condensate that is pure A. The separation technique is called 26/2/2021 fractional distillation. 24

The Distillation of Mixtures �Simple Distillation: Ø The vapour is withdrawn and condensed. Ø it is used to separate a volatile liquid from a nonvolatile solute or solid. �Fractional Distillation: Ø The boiling & condensation cycle is repeated successively. Ø It is used to separate volatile liquids. �Theoretical plates – the efficiency of a fractionating column. Ø The number of effective vaporization & condensation steps that are required to achieve a condensate of given composition from a given distillate. 2/26/2021 25

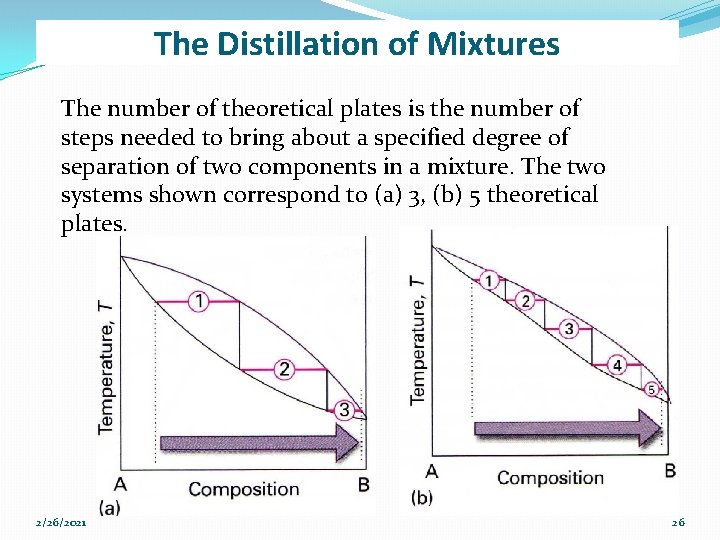
The Distillation of Mixtures The number of theoretical plates is the number of steps needed to bring about a specified degree of separation of two components in a mixture. The two systems shown correspond to (a) 3, (b) 5 theoretical plates. 2/26/2021 26

The Distillation of Mixtures �Azeotropes Ø A maximum in a phase diagram may occur when the favourable interaction between A & B molecules reduce the vapour pressure of the mixture below the ideal value. In effect, the A-B interactions stabilize the liquid. ü E. g. trichloromethane/propanone, ü hydrochloric acid/water & ü nitric acid/water mixtures. 2/26/2021 27

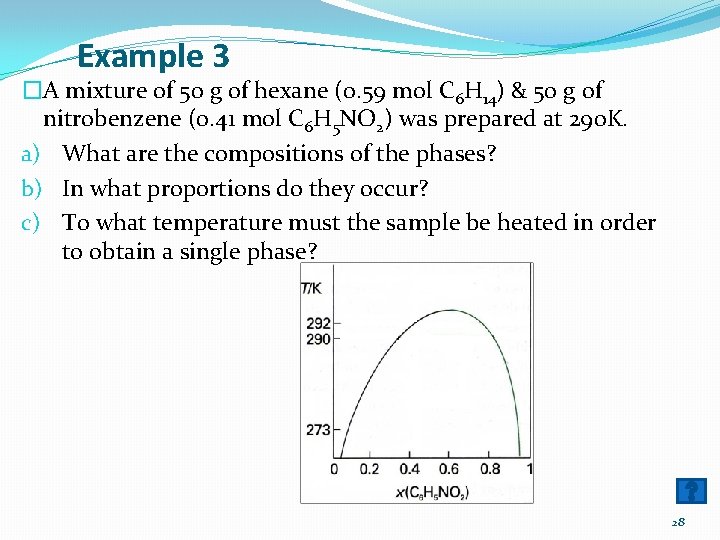
Example 3 �A mixture of 50 g of hexane (0. 59 mol C 6 H 14) & 50 g of nitrobenzene (0. 41 mol C 6 H 5 NO 2) was prepared at 290 K. a) What are the compositions of the phases? b) In what proportions do they occur? c) To what temperature must the sample be heated in order to obtain a single phase? 28

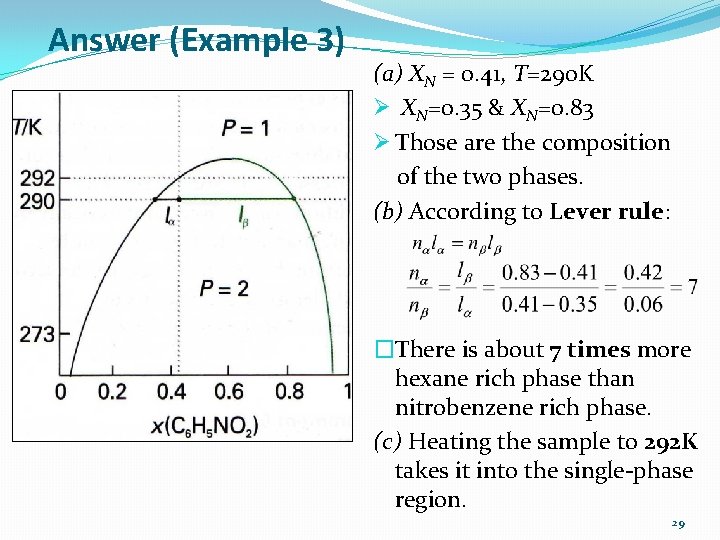
Answer (Example 3) (a) XN = 0. 41, T=290 K Ø XN=0. 35 & XN=0. 83 Ø Those are the composition of the two phases. (b) According to Lever rule: �There is about 7 times more hexane rich phase than nitrobenzene rich phase. (c) Heating the sample to 292 K takes it into the single-phase region. 29