Ternary Compounds Ternary compounds are those containing three






- Slides: 22

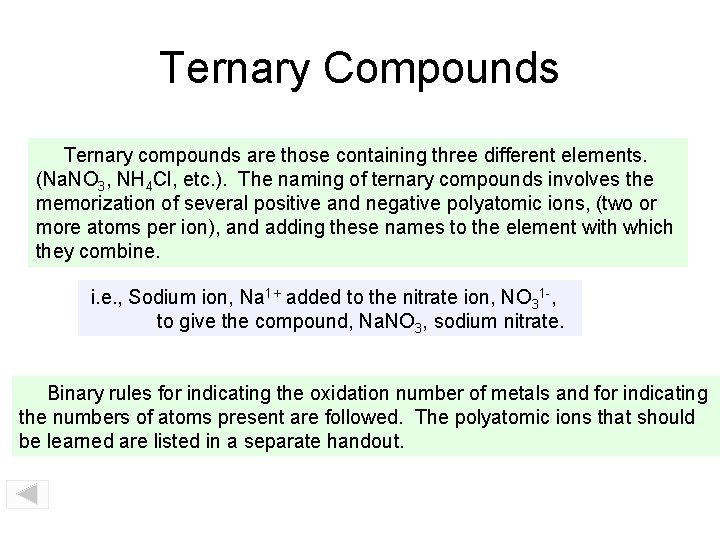
Ternary Compounds Ternary compounds are those containing three different elements. (Na. NO 3, NH 4 Cl, etc. ). The naming of ternary compounds involves the memorization of several positive and negative polyatomic ions, (two or more atoms per ion), and adding these names to the element with which they combine. i. e. , Sodium ion, Na 1+ added to the nitrate ion, NO 31 -, to give the compound, Na. NO 3, sodium nitrate. Binary rules for indicating the oxidation number of metals and for indicating the numbers of atoms present are followed. The polyatomic ions that should be learned are listed in a separate handout.

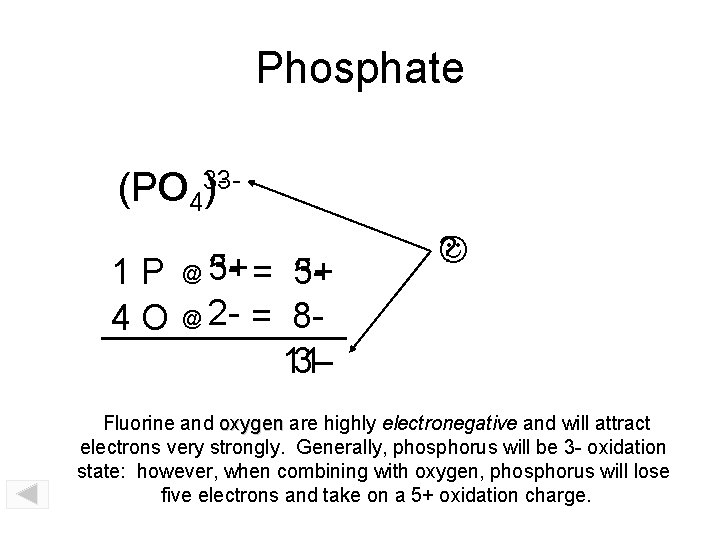
Phosphate (PO PO 43)31 P 4 O 5+ = 335+ @ 2 - = 8113@ ? Fluorine and oxygen are highly electronegative and will attract electrons very strongly. Generally, phosphorus will be 3 - oxidation state: however, when combining with oxygen, phosphorus will lose five electrons and take on a 5+ oxidation charge.

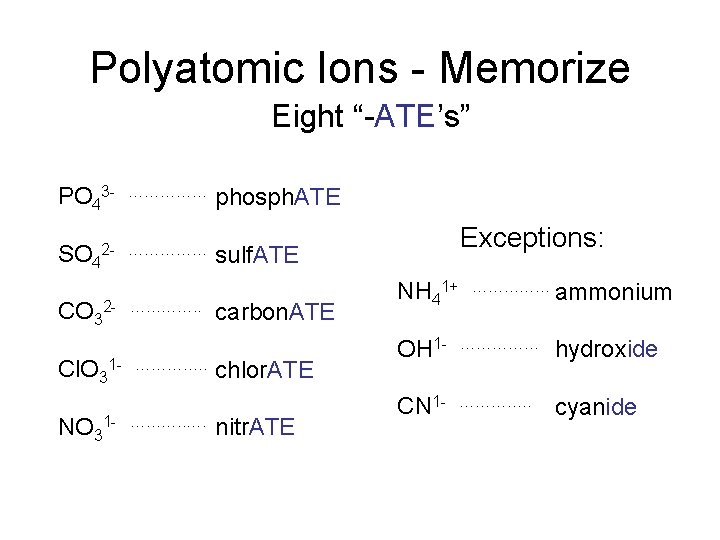
Polyatomic Ions - Memorize Eight “-ATE’s” PO 43 SO 4 …………… 2 - …………… CO 32 Cl. O 3 NO 3 …………. . 1 - ………. . …. phosphate phosph. ATE Exceptions: sulfate sulf. ATE carbonate carbon. ATE chlorate chlor. ATE nitrate nitr. ATE NH 41+ …………… ammonium OH 1 - …………… hydroxide CN 1 - …………. . cyanide

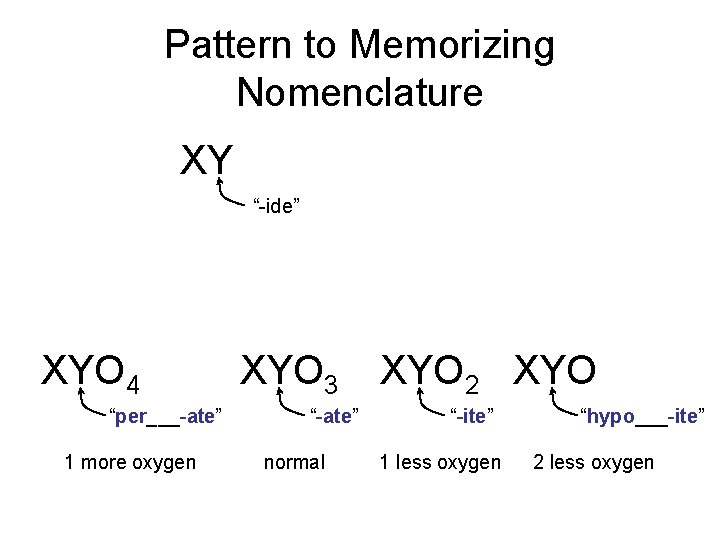
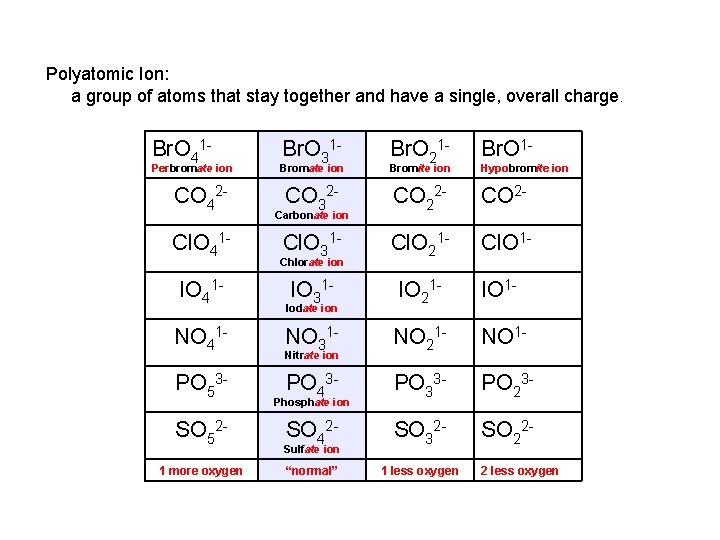
Pattern to Memorizing Nomenclature XY “-ide” XYO 4 “per___-ate” 1 more oxygen XYO 3 “-ate” normal XYO 2 XYO “-ite” 1 less oxygen “hypo___-ite” 2 less oxygen

Polyatomic Ion: a group of atoms that stay together and have a single, overall charge. Br. O 41 - Perbromate ion CO 42 Cl. O 41 IO 41 NO 41 PO 53 SO 521 more oxygen Br. O 31 - Br. O 1 - Bromate ion Br. O 21 - Bromite ion CO 32 - CO 2 - Cl. O 31 - Cl. O 21 - Cl. O 1 - IO 31 - IO 21 - IO 1 - NO 31 - NO 21 - NO 1 - PO 43 - PO 33 - PO 23 - SO 42 - SO 32 - SO 22 - “normal” 1 less oxygen Carbonate ion Chlorate ion Iodate ion Nitrate ion Phosphate ion Sulfate ion Hypobromite ion 2 less oxygen

Polyatomic Ion: a group of atoms that stay together and have a single, overall charge. Br. O 41 - Perbromate ion CO 42 Cl. O 41 IO 41 NO 41 PO 53 SO 521 more oxygen Br. O 31 - Br. O 1 - Bromate ion Br. O 21 - Bromite ion CO 32 - CO 2 - Cl. O 31 - Cl. O 21 - Cl. O 1 - IO 31 - IO 21 - IO 1 - NO 31 - NO 21 - NO 1 - PO 43 - PO 33 - PO 23 - SO 42 - SO 32 - SO 22 - “normal” 1 less oxygen Carbonate ion Chlorate ion Iodate ion Nitrate ion Phosphate ion Sulfate ion Hypobromite ion 2 less oxygen

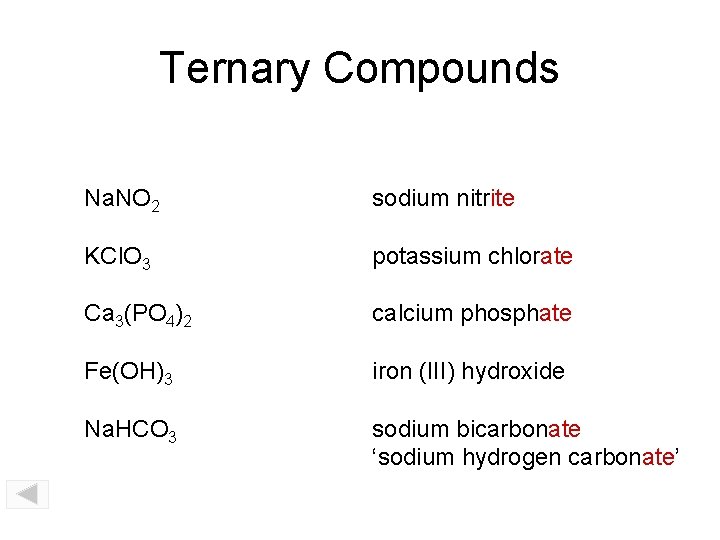
Ternary Compounds Na. NO 2 sodium nitrite KCl. O 3 potassium chlorate Ca 3(PO 4)2 calcium phosphate Fe(OH)3 iron (III) hydroxide Na. HCO 3 sodium bicarbonate ‘sodium hydrogen carbonate’

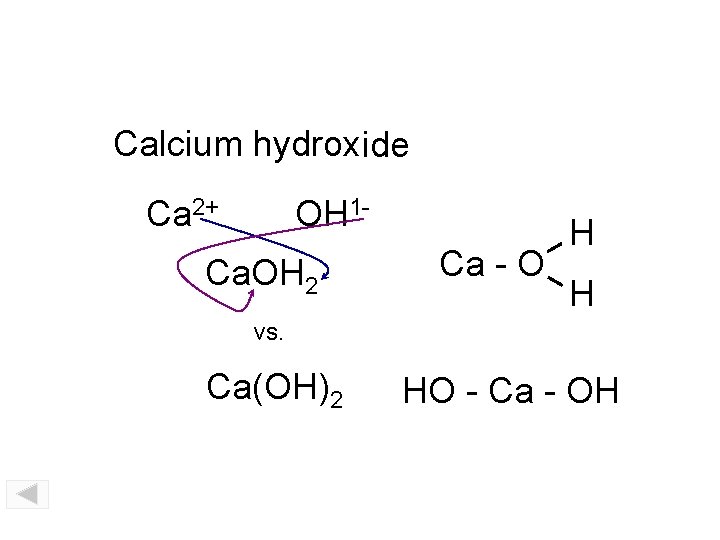
Calcium hydroxide Ca 2+ OH 1 - Ca. OH 2 Ca - O H H vs. Ca(OH)2 HO - Ca - OH

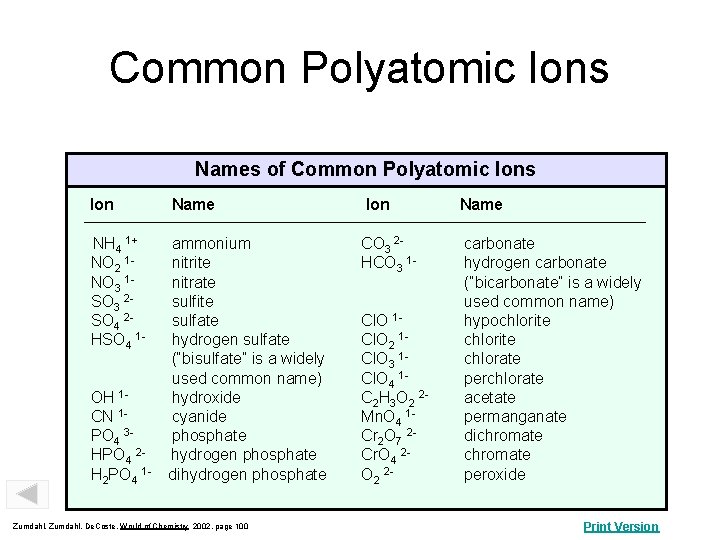
Common Polyatomic Ions Names of Common Polyatomic Ions Ion Name NH 4 1+ NO 2 1 NO 3 1 SO 3 2 SO 4 2 HSO 4 1 - ammonium nitrite nitrate sulfite sulfate hydrogen sulfate (“bisulfate” is a widely used common name) hydroxide cyanide phosphate hydrogen phosphate dihydrogen phosphate CO 3 2 HCO 3 1 - carbonate hydrogen carbonate (“bicarbonate” is a widely used common name) hypochlorite chlorate perchlorate acetate permanganate dichromate peroxide OH 1 CN 1 PO 4 3 HPO 4 2 H 2 PO 4 1 - Zumdahl, De. Coste, World of Chemistry 2002, page 100 Cl. O 1 Cl. O 2 1 Cl. O 3 1 Cl. O 4 1 C 2 H 3 O 2 2 Mn. O 4 1 Cr 2 O 7 2 Cr. O 4 2 O 2 2 - Print Version

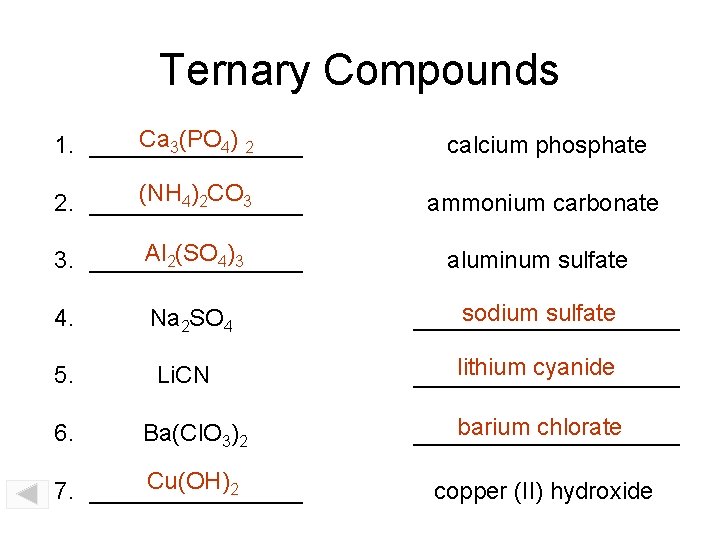
Ternary Compounds Ca 3(PO 4) 2 1. ________ calcium phosphate (NH 4)2 CO 3 2. ________ ammonium carbonate Al 2(SO 4)3 3. ________ aluminum sulfate 4. Na 2 SO 4 sodium sulfate __________ 5. Li. CN lithium cyanide __________ 6. Ba(Cl. O 3)2 Cu(OH)2 7. ________ barium chlorate __________ copper (II) hydroxide

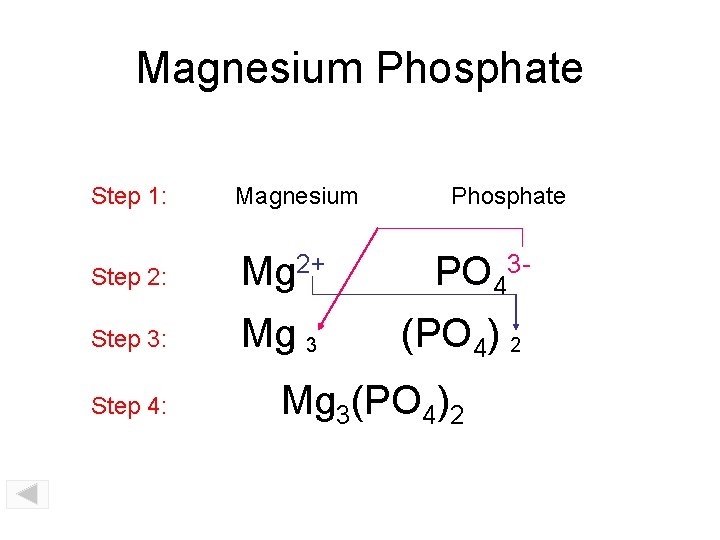
Magnesium Phosphate Step 1: Magnesium Step 2: Mg 2+ PO 43 - Step 3: Mg 3 (PO 4) 2 Step 4: Phosphate Mg 3(PO 4)2

variable Ir 2+, 3+, 4+, 6+ Ir F 2(Cr 3 2 O 7 )3 iridium (III) dichromate fluoride Ca S (OH)2 calcium hydroxide sulfide Ti S (Cr. O 2 4 )2 titanium (IV) chromate sulfide variable Ti 3+, 4+ Pt Cl (CH 2 3 COO)2 platinum (II) acetate chloride variable Pt 2+, 4+ Ba. Br (Br. O 2 3 )2 barium bromide bromate fixed Ba 2+ Sr 3 SO P 2 4 strontium sulfate phosphide fixed Sr 2+ KF CN potassium cyanide fluoride fixed K 1+ Zn I(NO 2 2 )2 zinc nitrite iodide fixed Zn 2+ Mn Cl (Cl. O 4 3 )4 manganese (IV) chlorate chloride variable Mn 2, 3, 4, 6, 7+ Au PO 2 O 34 gold (III) phosphate oxide Na 3 NO P 3 sodium nitrate phosphide fixed Ca 2+ variable Au 1+, 3+ fixed Na 1+

variable Ir 2+, 3+, 4+, 6+ Ir F 3 iridium (III) fluoride Ca S calcium sulfide Ti S 2 titanium (IV) sulfide variable Ti 3+, 4+ Pt Cl 2 platinum (II) chloride variable Pt 2+, 4+ Ba. Br 2 barium bromide fixed Ba 2+ Sr 3 P 2 strontium phosphide fixed Sr 2+ KF potassium fluoride fixed K 1+ Zn I 2 zinc iodide fixed Zn 2+ Mn Cl 4 manganese (IV) chloride variable Mn 2, 3, 4, 6, 7+ Au 2 O 3 gold (III) oxide Na 3 P sodium phosphide fixed Ca 2+ variable Au 1+, 3+ fixed Na 1+

variable Ir 2+, 3+, 4+, 6+ Ir 2(Cr 2 O 7)3 iridium (III) dichromate Ca (OH)2 calcium hydroxide Ti (Cr. O 4)2 titanium (IV) chromate variable Ti 3+, 4+ Pt (CH 3 COO)2 platinum (II) acetate variable Pt 2+, 4+ Ba(Br. O 3)2 barium bromate fixed Ba 2+ Sr 3 SO 4 strontium sulfate fixed Sr 2+ KCN potassium cyanide fixed K 1+ Zn (NO 2)2 zinc nitrite fixed Zn 2+ Mn (Cl. O 3)4 manganese (IV) chlorate variable Mn 2, 3, 4, 6, 7+ Au PO 4 gold (III) phosphate Na NO 3 sodium nitrate fixed Ca 2+ variable Au 1+, 3+ fixed Na 1+

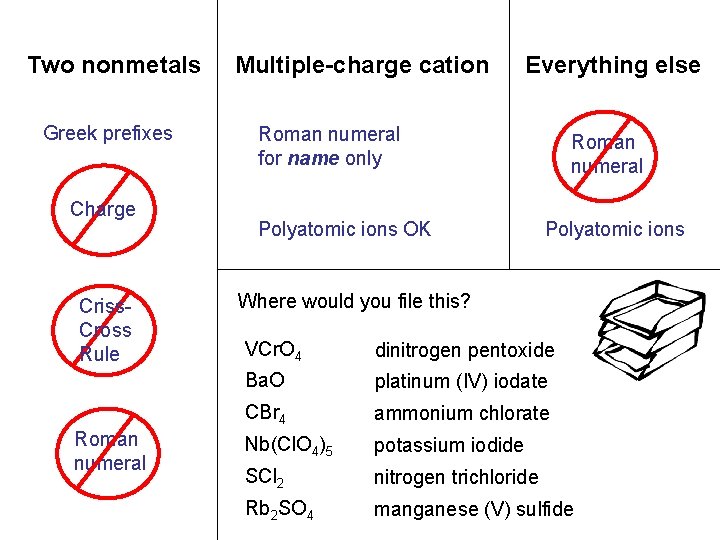
Two nonmetals Multiple-charge cation Everything else carbon sulfur. N tetrabromide dichloride NCl O 35 2 vanadium niobium. Mn Pt(IO (V) (II) perchlorate )4 2 S 53 chromate rubidium sulfate NH 4 KI Cl. O barium oxide 3 Greek prefixes Charge Criss. Cross Rule Roman numeral for name only Polyatomic ions OK Roman numeral Polyatomic ions OK Where would you file this? VCr. O 4 dinitrogen pentoxide Ba. O platinum (IV) iodate CBr 4 ammonium chlorate Nb(Cl. O 4)5 potassium iodide SCl 2 nitrogen trichloride Rb 2 SO 4 manganese (V) sulfide

Two nonmetals Greek prefixes Charge Criss. Cross Rule Roman numeral Multiple-charge cation Everything else Roman numeral for name only Polyatomic ions OK Roman numeral Polyatomic ions Where would you file this? VCr. O 4 dinitrogen pentoxide Ba. O platinum (IV) iodate CBr 4 ammonium chlorate Nb(Cl. O 4)5 potassium iodide SCl 2 nitrogen trichloride Rb 2 SO 4 manganese (V) sulfide

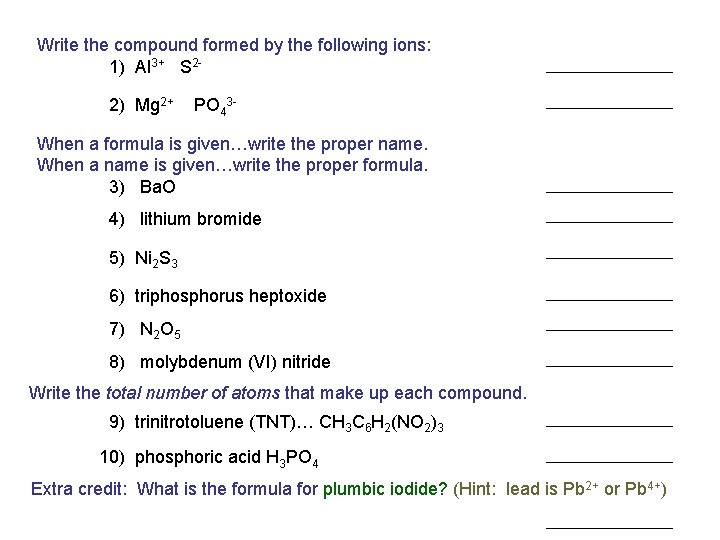
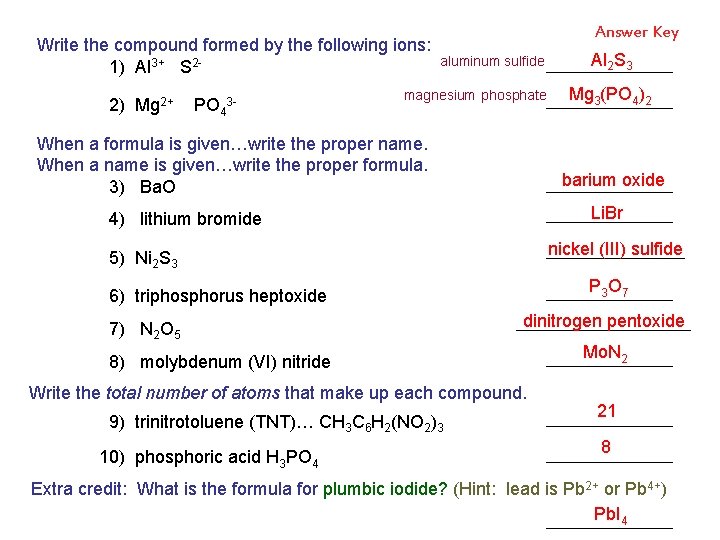
Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O 4) lithium bromide 5) Ni 2 S 3 6) triphosphorus heptoxide 7) N 2 O 5 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+)

Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O POP QUIZ 4) lithium bromide 5) Ni 2 S 3 6) triphosphorus heptoxide 7) N 2 O 5 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+)

Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - Answer Key aluminum sulfide magnesium phosphate When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O Li. Br nickel (III) sulfide 5) Ni 2 S 3 P 3 O 7 6) triphosphorus heptoxide dinitrogen pentoxide 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Mg 3(PO 4)2 barium oxide 4) lithium bromide 7) N 2 O 5 Al 2 S 3 Mo. N 2 21 8 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+) Pb. I 4

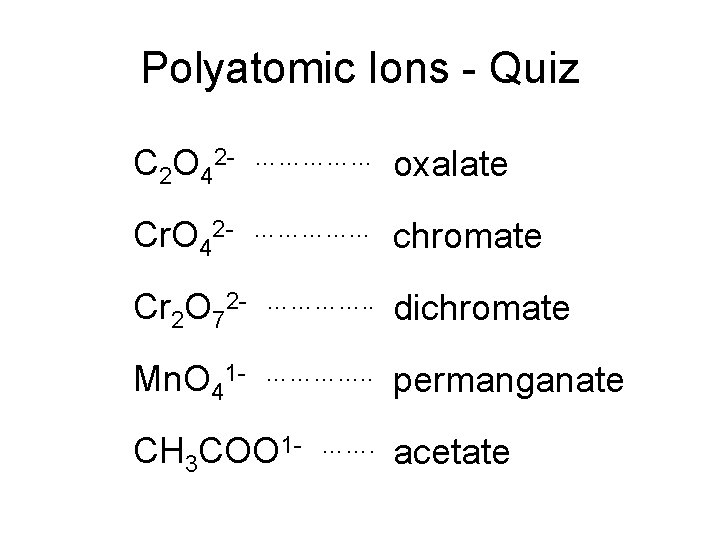
Polyatomic Ions - Quiz C 2 O 42 - …………… oxalate Cr. O 42 - …………… chromate Cr 2 O 72 - …………. . dichromate Mn. O 41 - …………. . permanganate CH 3 COO 1 - ……. acetate

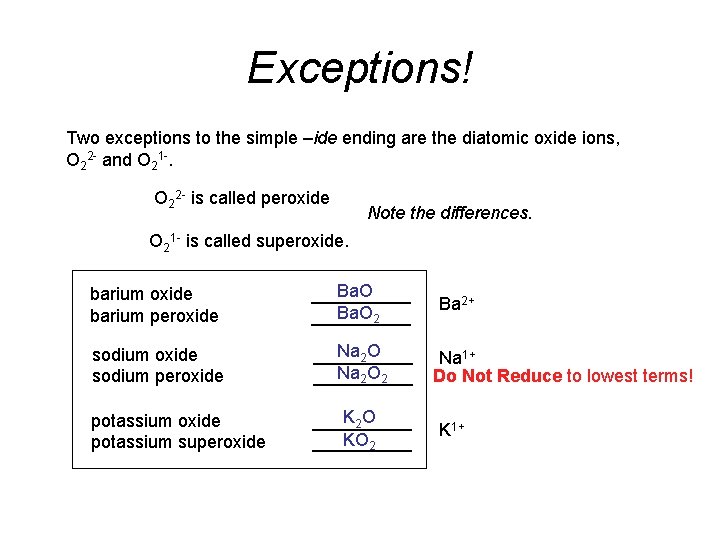
Exceptions! Two exceptions to the simple –ide ending are the diatomic oxide ions, O 22 - and O 21 -. O 22 - is called peroxide Note the differences. O 21 - is called superoxide. barium oxide barium peroxide Ba. O _____ Ba. O 2 _____ sodium oxide sodium peroxide Na 2 O _____ Na 2 O 2 _____ potassium oxide potassium superoxide K 2 O _____ KO 2 _____ Ba 2+ Na 1+ Do Not Reduce to lowest terms! K 1+

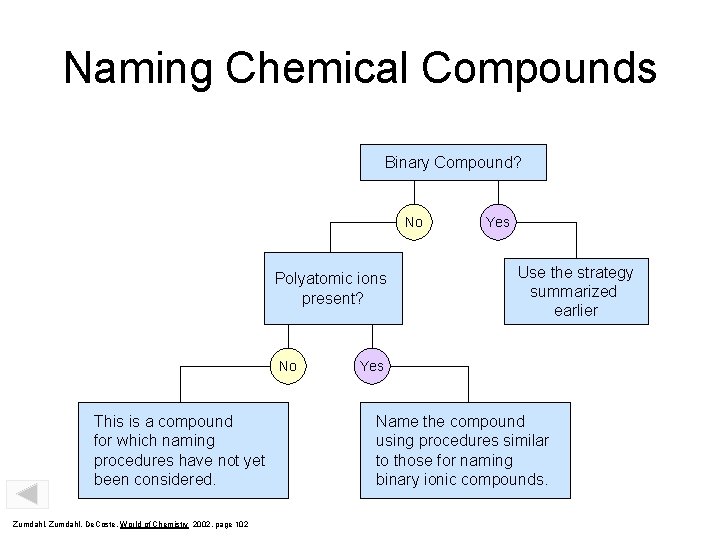
Naming Chemical Compounds Binary Compound? No Polyatomic ions present? No This is a compound for which naming procedures have not yet been considered. Zumdahl, De. Coste, World of Chemistry 2002, page 102 Yes Use the strategy summarized earlier Yes Name the compound using procedures similar to those for naming binary ionic compounds.