INTRODUCTION Organometallic compounds are the Compounds containing a






- Slides: 23

INTRODUCTION Organometallic compounds are the Compounds containing a carbon –metal bond. Since lithium is a metal , so it forms an organo-metallic compound. The compounds formed by carbon– lithium bonds are called organolithium compounds e. g. CH 3 -Li ‘CH 3’ is an organic group ‘Li’ is the metallic group Dr. HENRY GILMAN (Father of organometallic chemistry)

DISCOVERY Organolithiumcompounds, discovered by Wilhelm Schlenk in 1917. But only since the 1950 th, based on the pioneering work of Georg Wittig and Henry Gilman, organometallic reagents became a routinely used tool in the synthetic organic laboratory. Dr. WILHELM SCHLENK (German chemist, (1879 -1943)

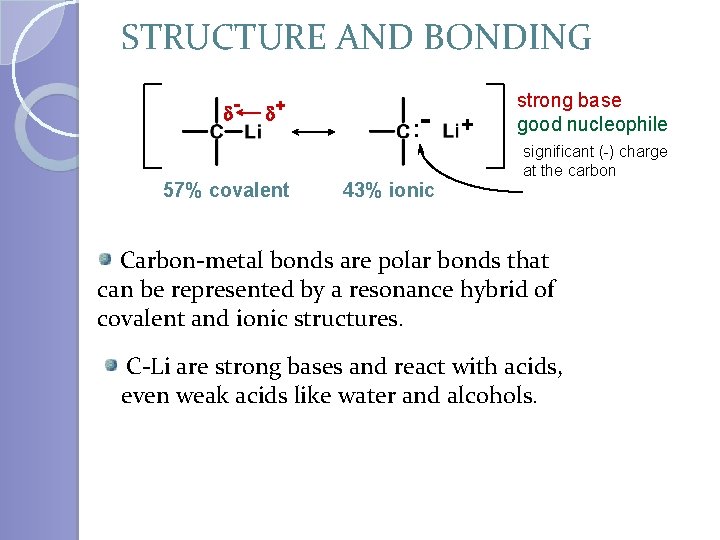
STRUCTURE AND BONDING d- d+ 57% covalent : 43% ionic + strong base good nucleophile significant (-) charge at the carbon Carbon-metal bonds are polar bonds that can be represented by a resonance hybrid of covalent and ionic structures. C-Li are strong bases and react with acids, even weak acids like water and alcohols.

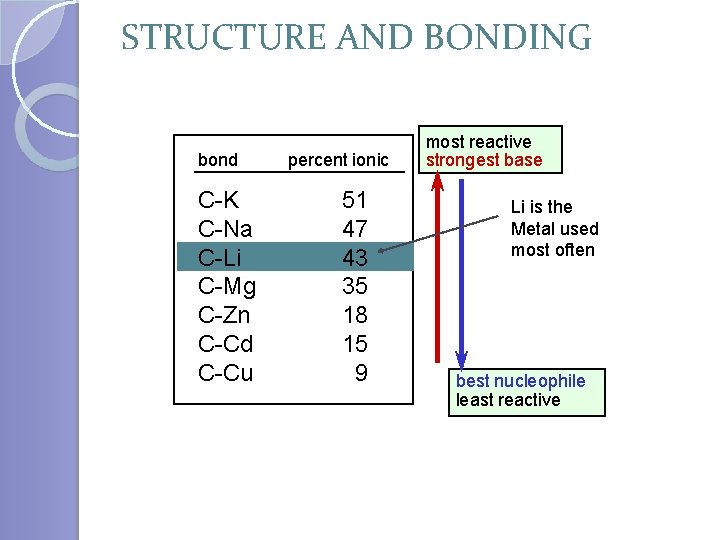
STRUCTURE AND BONDING bond C-K C-Na C-Li C-Mg C-Zn C-Cd C-Cu percent ionic 51 47 43 35 18 15 9 most reactive strongest base Li is the Metal used most often best nucleophile least reactive

PREPARATION OF ORGANOLITHIUM COMPOUNDS The organometallic compounds can be prepared by following methods: By Halogen Metal Exchange From Terminal Alkynes By Trans Metalation/ Metal-Metal Exchange By Directed Metalation/ Ortho Metalation

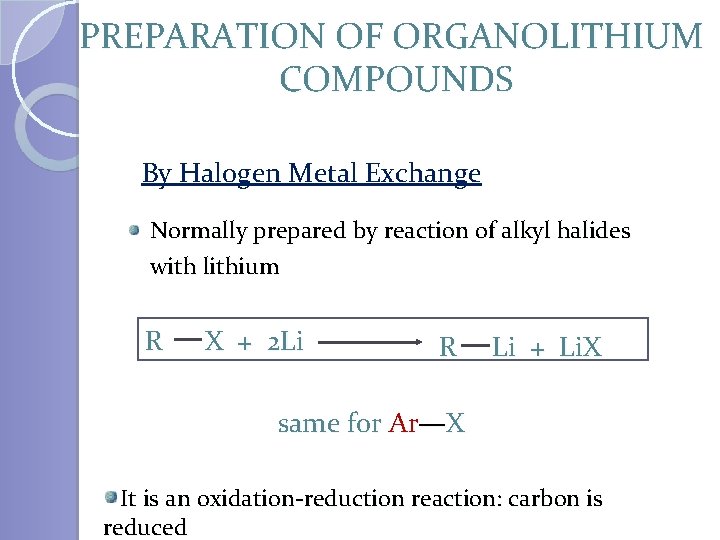
PREPARATION OF ORGANOLITHIUM COMPOUNDS By Halogen Metal Exchange Normally prepared by reaction of alkyl halides with lithium R X + 2 Li R Li + Li. X same for Ar—X It is an oxidation-reduction reaction: carbon is reduced

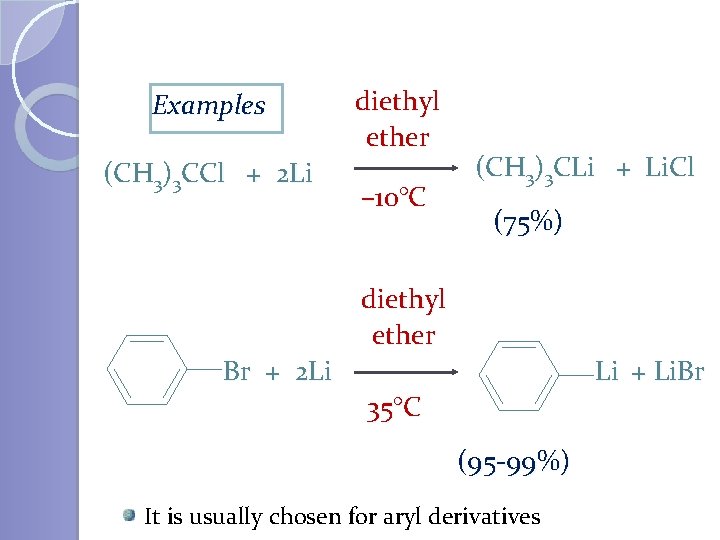
Examples (CH 3)3 CCl + 2 Li diethyl ether – 10°C (CH 3)3 CLi + Li. Cl (75%) diethyl ether Br + 2 Li Li + Li. Br 35°C (95 -99%) It is usually chosen for aryl derivatives

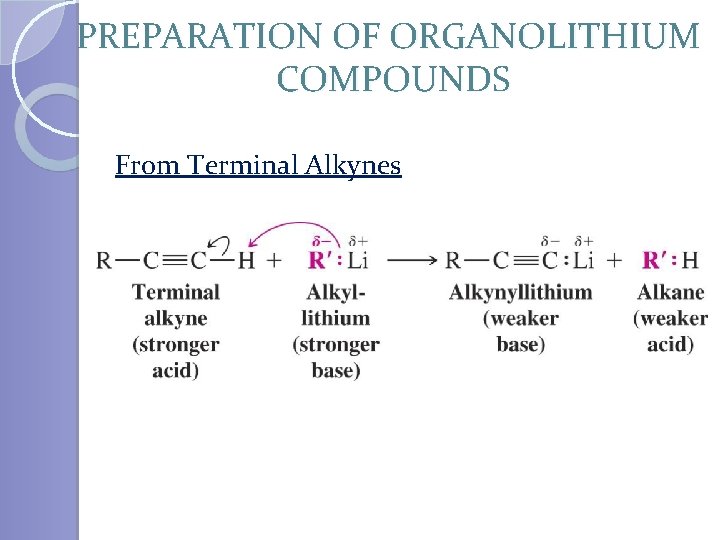
PREPARATION OF ORGANOLITHIUM COMPOUNDS From Terminal Alkynes

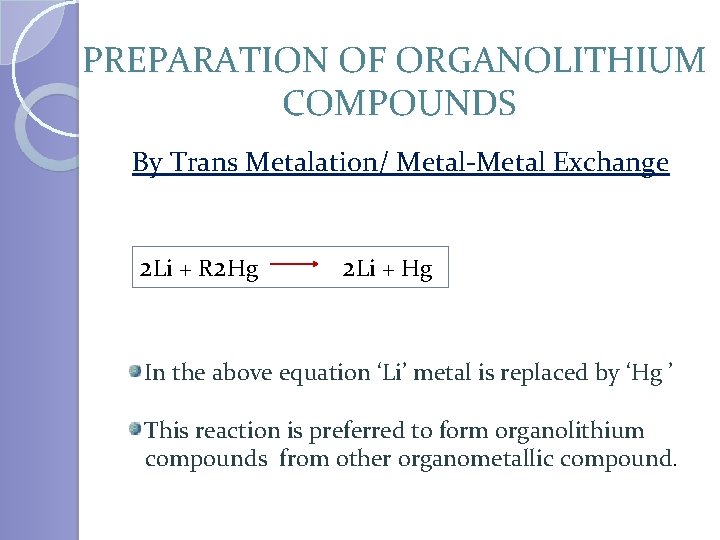
PREPARATION OF ORGANOLITHIUM COMPOUNDS By Trans Metalation/ Metal-Metal Exchange 2 Li + R 2 Hg 2 Li + Hg In the above equation ‘Li’ metal is replaced by ‘Hg ’ This reaction is preferred to form organolithium compounds from other organometallic compound.

PREPARATION OF ORGANOLITHIUM COMPOUNDS By Directed Metalation/ Ortho Metalation DMG + RLi Li + R-H Metallation of an aromatic ring near a substituent, which acts as a “Directed Metallation Group”, is called “Ortho. Metallation”. They have in common the ability to coordinate the approaching cation (= lithium-ion) and/or to increase the acidity of the orthohydrogen

PROPERTIES OF ORGANOLITHIUM COMPOUNDS Physical properties Highly polar due to most electro positivity of lithium group. Volatile in nature, easily absorbs moisture. Solubility in Hydrocarbons. More complex bonding. Forms covalent compounds due to vacant ‘p’ orbital Polarity of Bonds

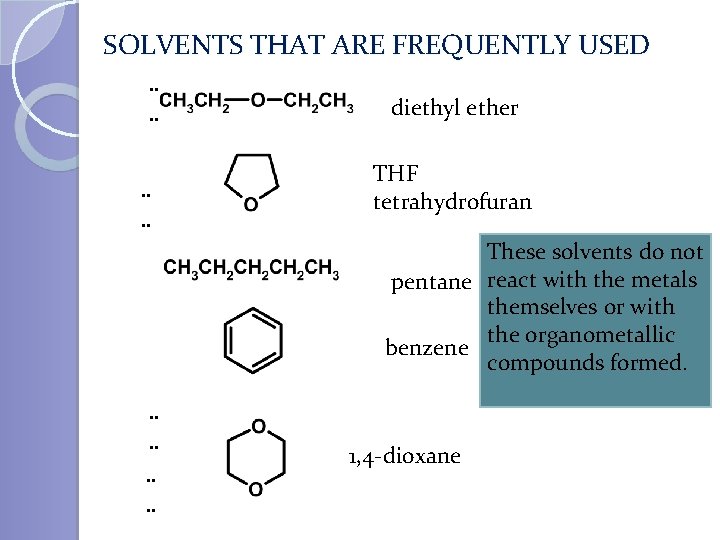
SOLVENTS THAT ARE FREQUENTLY USED. . . . diethyl ether THF tetrahydrofuran These solvents do not pentane react with the metals themselves or with the organometallic benzene compounds formed. . 1, 4 -dioxane

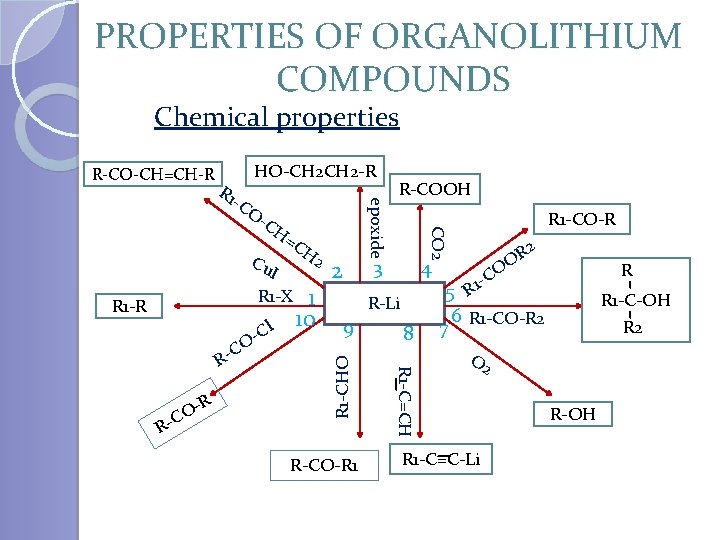
PROPERTIES OF ORGANOLITHIUM COMPOUNDS Chemical properties R-CO-CH=CH-R -C O =C Cu R 1 -R l C O- R O C R- 3 4 R-Li 9 R-CO-R 1 8 R 1 -C=CH C R- 1 10 2 R 1 -CHO I R 1 -X H 2 R-COOH R 1 -CO-R CO 2 -C H epoxide R 1 HO-CH 2 -R 2 R O -C 1 5 R 6 R 1 -CO-R 2 R 1 -C-OH R 2 7 O 2 R 1 -C=C-Li R-OH

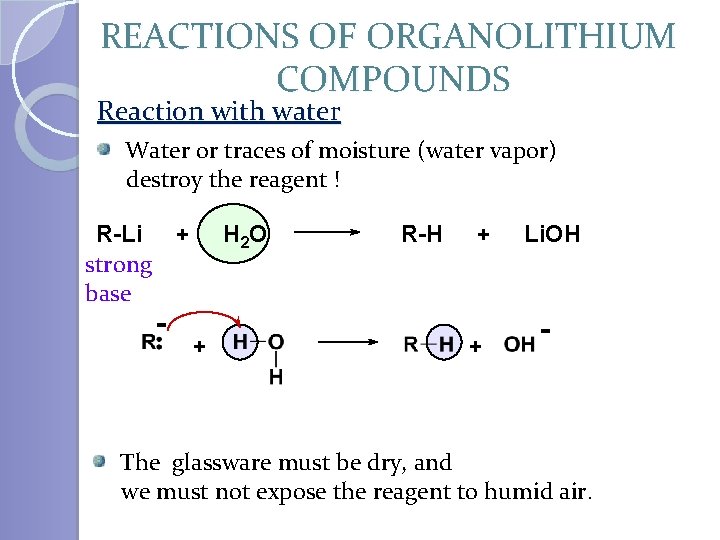
REACTIONS OF ORGANOLITHIUM COMPOUNDS Reaction with water Water or traces of moisture (water vapor) destroy the reagent ! R-Li strong base + - H 2 O + R-H + + Li. OH - The glassware must be dry, and we must not expose the reagent to humid air.

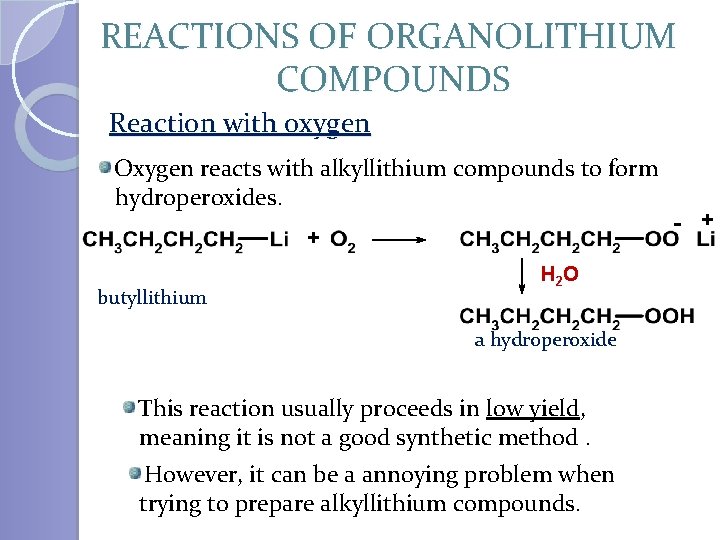
REACTIONS OF ORGANOLITHIUM COMPOUNDS Reaction with oxygen Oxygen reacts with alkyllithium compounds to form hydroperoxides. + butyllithium H 2 O a hydroperoxide This reaction usually proceeds in low yield, meaning it is not a good synthetic method. However, it can be a annoying problem when trying to prepare alkyllithium compounds. - +

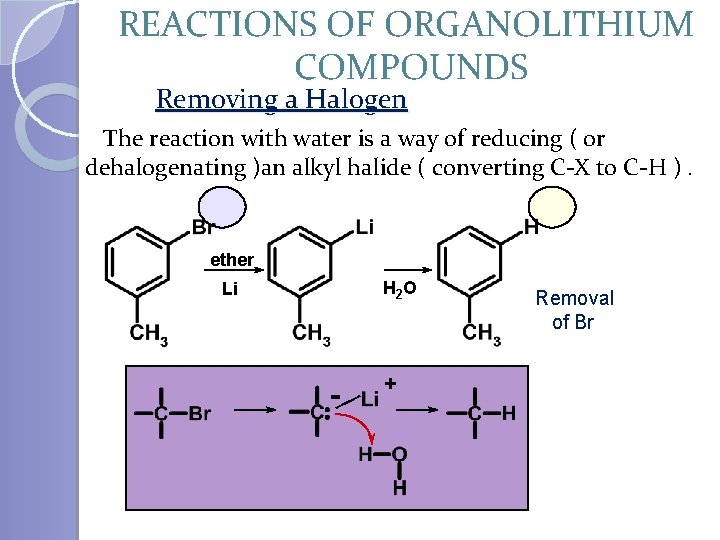
REACTIONS OF ORGANOLITHIUM COMPOUNDS Removing a Halogen The reaction with water is a way of reducing ( or dehalogenating )an alkyl halide ( converting C-X to C-H ). ether H 2 O Li - + Removal of Br

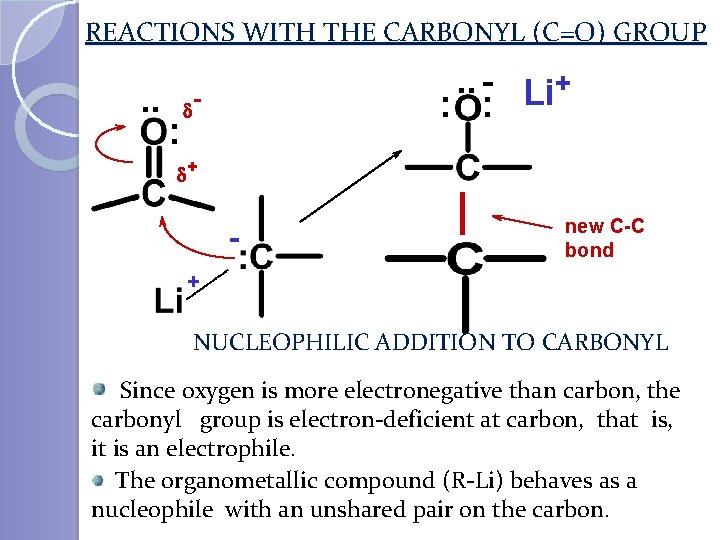
REACTIONS WITH THE CARBONYL (C=O) GROUP . . : . . - Li+ : : d d+ - new C-C bond + NUCLEOPHILIC ADDITION TO CARBONYL Since oxygen is more electronegative than carbon, the carbonyl group is electron-deficient at carbon, that is, it is an electrophile. The organometallic compound (R-Li) behaves as a nucleophile with an unshared pair on the carbon.

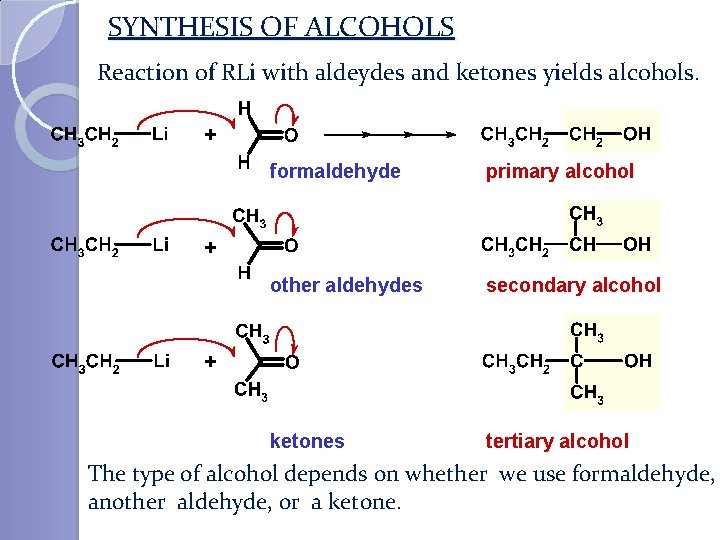
SYNTHESIS OF ALCOHOLS Reaction of RLi with aldeydes and ketones yields alcohols. + formaldehyde primary alcohol other aldehydes secondary alcohol ketones tertiary alcohol + + The type of alcohol depends on whether we use formaldehyde, another aldehyde, or a ketone.

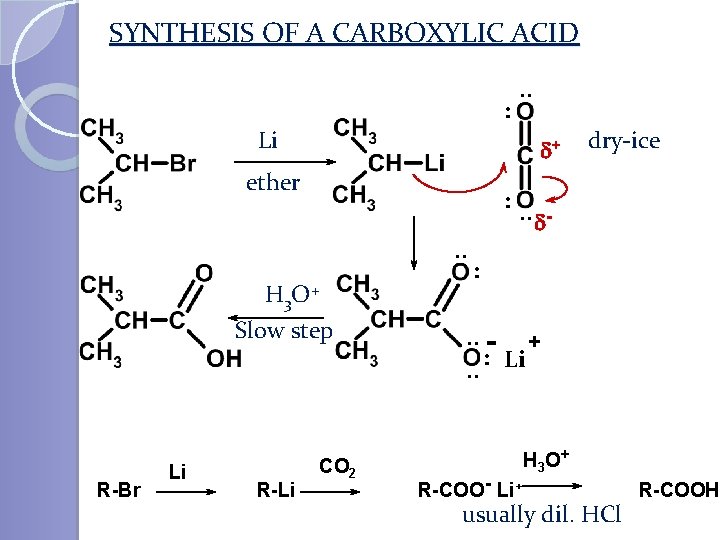
SYNTHESIS OF A CARBOXYLIC ACID : . . Li d+ ether : . . d. . H 3 O + Slow step R-Br Li R-Li dry-ice CO 2 : . . - + : . . Li R-COO - Li+ H 3 O + usually dil. HCl R-COOH

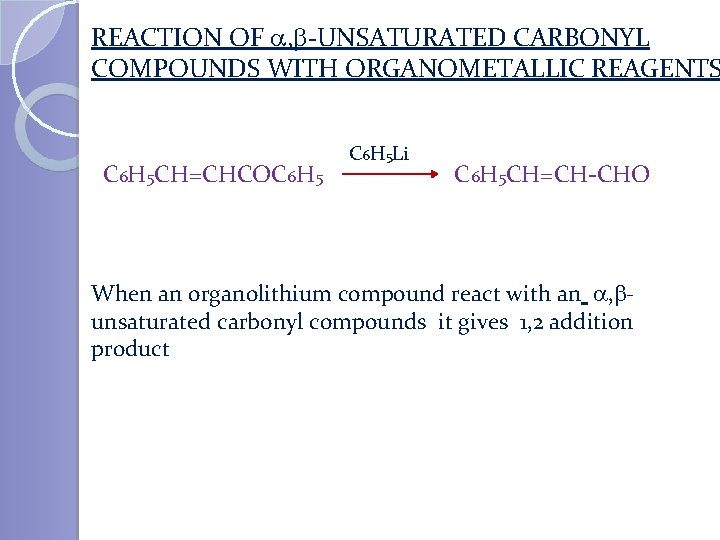
REACTION OF , -UNSATURATED CARBONYL COMPOUNDS WITH ORGANOMETALLIC REAGENTS C 6 H 5 CH=CHCOC 6 H 5 Li C 6 H 5 CH=CH-CHO When an organolithium compound react with an , unsaturated carbonyl compounds it gives 1, 2 addition product

RECENT DEVELOPMENTS Lithium hexamethyldisilazide (LHMDS) lithium amide, 30 % solution in hexane Mol. Formula: C 6 H 18 Li. NSi 2 Application: Selective low nucleophilic base for e. g. enolisations Appearance: yellowish solution Physical Properties: Molecular weight: 167. 33 Density: 0. 71 g/ccm (at 20°C) Boiling point: 60 – 80 °C for Hexane

LIMITATIONS OF ORGANOLITHIUM COMPOUNDS Highly volatile , so not used in mild reaction. It easily absorbs moisture , because of which it is not kept in open envoirnment. These compounds are harmful for skin. so, we should use gloves before using it

H T N A Y K U O