THERMODYNAMIC DIAGRAMS 10282020 1 AEROLOGICAL DIAGRAMS DIAGRAMS USED













- Slides: 22

THERMODYNAMIC DIAGRAMS 10/28/2020 1

AEROLOGICAL DIAGRAMS • DIAGRAMS USED FOR THE STUDY OF THERMODYNAMIC PROCESSES OF THE ATMOSPHERE SUCH AS DRY ADIABATIC, ISOBARIC, PSUDOADIABATIC ETC, ARE OFTEN REFERRED TO AS AEROLOGICAL DIAGRAMS. • THESE DIAGRAMS ARE DEVISED TO INDICATE, IN PICTORIAL MANNER, THE DISTRIBUTION OF TEMPERATURE AND MOISTURE ABOVE A STATION. 10/28/2020 2

10/28/2020 3

10/28/2020 4

COORDINATES • TEMPERATURES AND HUMIDITIES ARE USUALLY DETERMINED AT FIXED PRESSURES. • SINCE IT IS KNOWN THAT THE LOGARITHM OF PRESSURE IS CLOSELY RELATED TO THE ALTITUDE, ‘TEMPERATURE’ AND ‘LOGARITHM OF PRESSURE’ ARE GENERALLY USED AS COORDINATES OF THERMODYNAMIC DIAGRAMS. 10/28/2020 5

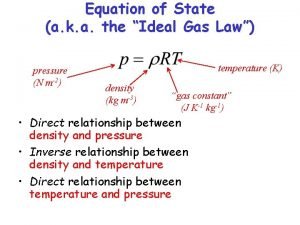
DESIRABLE PROPERTIES OF THERMODYNAMICS • THERE ARE FOUR DESIRABLE PROPERTIES WHICH PRACTICAL THERMODYNAMIC DIAGRAMS SHOULD POSSES: – THE AREA ENCLOSED BY THE LINES REPRESENTING ANY CYCLIC PROCESS SHOULD BE PROPORTIONAL TO THE CHANGE IN ENERGY OR THE WORK DONE DURING THE PROCESS. – AS MANY AS POSSIBLE OF THE LINES, REPRESENTING BASIC PROCESSESES, SHOULD BE STRAIGHT. – THE ANGLE BETWEEN THE ISOTHERMS (LINES OF EQUAL TEMPERATURE) AND THE DRY ADIABATS (LINES OF EQUAL POTENTIAL TEMPERATURE) SHOULD BE AS LARGE AS POSSIBLE. – IN THE LOWER ATMOSPHERE, THE DRY ADIABATS (INDICATING DRY ADIABATIC PROCESS) SHOULD MAKE AN APPRECIABLE ANGLE WITH THE SATURATION ADIABATS (INDICATING PSEUDO-ADIABATIC PROCESS). 10/28/2020 6

DESIRABLE PROPERTIES OF THERMODYNAMICS……. CONTD • FOR A FINITE PROCESS WITH INITIAL AND FINAL STATES DESIGNATED BY THE NUMBER 1 AND 2, THE WORK DONE PER UNIT MASS IS GIVEN BY THE INTEGRAL • THIS SUGGESTS THAT SPECIFIC VOLUME (α) AND PRESSURE (p) CUOLD BE USED AS COORDINATES TO SATISFY THE FIRST CRITERION. • IN PRACTICE, HOWEVER, THE ANGLE BETWEEN THE ISOTHERMS AND THE DRY ADIABATS IS QUITE SMALL. • HENCE, AN (α-p) DIAGRAM DOES NOT SATISFY THE THIRD CRITERION. 10/28/2020 7

THE TEPHIGRAM 10/28/2020 8

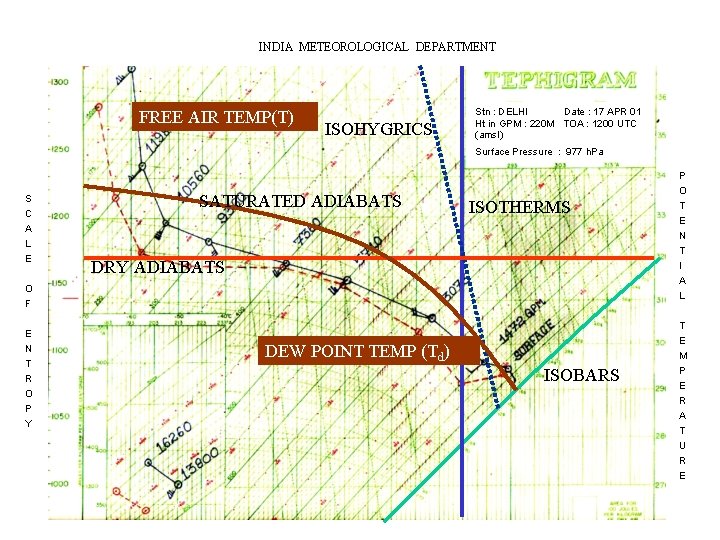
INDIA METEOROLOGICAL DEPARTMENT FREE AIR TEMP(T) ISOHYGRICS Stn : DELHI Date : 17 APR 01 Ht in GPM : 220 M TOA : 1200 UTC (amsl) Surface Pressure : 977 h. Pa S C A L E SATURATED ADIABATS ISOTHERMS DRY ADIABATS O F E N T R O P Y DEW POINT TEMP (Td) ISOBARS P O T E N T I A L T E M P E R A T U R E

USES OF T- GRAM • DETERMINING MET PARAMETERS • STUDYING THE FORCES WHICH COME INTO PLAY WHEN ATMOSPHERE IS SUBJECTED TO VETICAL MOTIONS • DETERMINING THE STABILITY OF THE ATMOSPHERE • PREDICTION OF CONVECTIVE PHENOMENON

FEW IMPORTANT DEFINITIONS • ADIABATIC PROCESSES. AN ADIABATIC PROCESS IS DEFINED AS ONE IN WHICH A SAMPLE OF GAS NEITHER GAINS HEAT FROM NOR LOSSES HEAT TO ITS SURROUNDINGS. NO TRANSFER OF HEAT TAKES PLACE I. E. , NO HEAT IS ADDED OR TAKEN AWAY FROM A SAMPLE OF GAS. • POTENTIAL TEMPERATURE THE AS DEFINED THAT A SAMPLE OF GAS WOULD HAVE IF IT WERE BROUGHT ADIABATICALLY FROM AN INITIAL STATE P AND T TO A PRESSURE OF 1000 HPA. • MIXING RATIO (r). IS THE RATIO OF THE MASS OF WATER VAPOUR (mv) TO THE MASS OF DRY AIR (md) IN A GIVEN SAMPLE OF MOIST AIR IS THE MIXING RATIO. • SATURATION MIXING RATIO (rs). IS THE MIXING RATIO OF THE SAMPLE OF AIR WHEN IT IS COMPLETELY SATURATED.

FEW IMPORTANT DEFINITIONS • RELATIVE HUMIDITY. IT IS THE RATIO OF THE AMOUNT OF WATER VAPOUR ACTUALLY PRESENT IN AIR TO THE AMOUNT OF WATER VAPOUR NECESSARY TO MAKE IT SATURATE AT THAT TEMPERATURE AND PRESSURE AND IS EXPRESSED IN PERCENTAGE. • DEW POINT TEMPERATURE (Td). IT IS THAT TEMPERATURE TO WHICH MOIST AIR MUST BE COOLED DURING A PROCESS IN WHICH P AND r REMAIN CONSTANT IN ORDER THAT THE AIR BECOMES JUST SATURATED WITH RESPECT TO WATER. IF AIR IS COOLED BELOW THE DEW POINT TEMPERATURE CONDENSATION TAKES PLACE AND DEW FORMS.

FEW IMPORTANT DEFINITIONS • DRY ADIABATIC LAPSE RATE. IT IS THE RATE AT WHICH THE PARCEL OF UNSATURATED AIRMASS COOLS OR WARMS UP WHEN PARCEL IS LIFTED UP OR BROUGHT DOWN ADIABATICALLY. • THE LIFTING CONDENSATION LEVEL (LCL). IT IS THE LEVEL TO WHICH A PARCEL OF AIR HAS TO BE LIFTED ADIABATICALLY TO MAKE IT SATURATED. AT THE LCL, THE MIXING RATIO BECOMES EQUAL TO THE SATURATION-MIXING RATIO. • CONVECTIVE CONDENSATION LEVEL (CCL). IT IS THE LEVEL TO WHICH A PARCEL OF AIR IF HEATED SUFFICIENTLY FROM BELOW WILL RISE ADIABATICALLY UNTIL IT IS JUST SATURATED. • CONVECTIVE TEMPERATURE. IT IS THE SURFACE TEMPERATURE THAT MUST BE ATTAINED TO START THE FORMATION OF CONVECTIVE CLOUDS BY SOLAR HEATING OF THE SURFACE AIR.

FEW IMPORTANT DEFINITIONS • LEVEL OF FREE CONVECTION (LFC). IT IS THE LEVEL TO WHICH A PARCEL OF AIR, LIFTED DRYADIABATICALLY UNTIL SATURATED AND SATURATED ADIABATICALLY THEREAFTER UNTIL IT BECOMES WARMER (LESS DENSE) THAN THE SURROUNDING AIR. THE PARCEL WILL THEN CONTINUE TO RISE FREELY ABOVE THIS LEVEL UNTIL BECOMES COLDER (MORE DENSE) THAN THE SURROUNDING AIR. • EQUILIBRIUM LEVEL (EL). IT IS THE LEVEL WHERE THE BUOYANTLY RISING PARCEL OF AIR ATTAINS THE TEMPERATURE OF THE ENVIRONMENT.

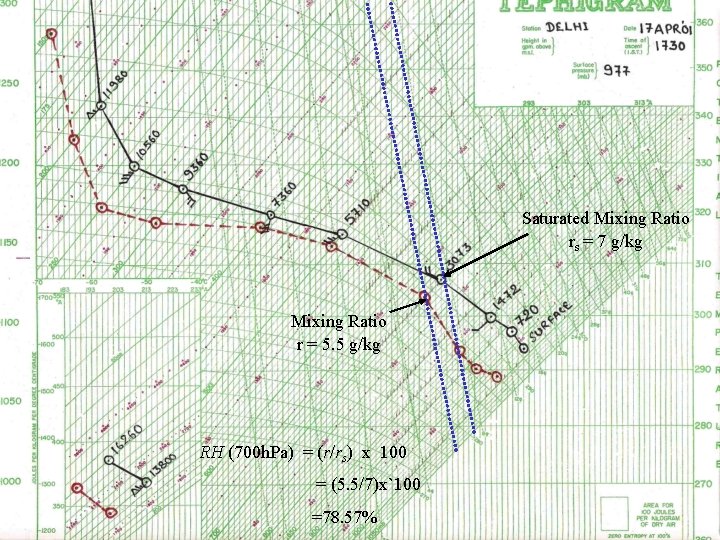
Saturated Mixing Ratio rs = 7 g/kg Mixing Ratio r = 5. 5 g/kg RH (700 h. Pa) = (r/rs) x 100 = (5. 5/7)x`100 =78. 57%

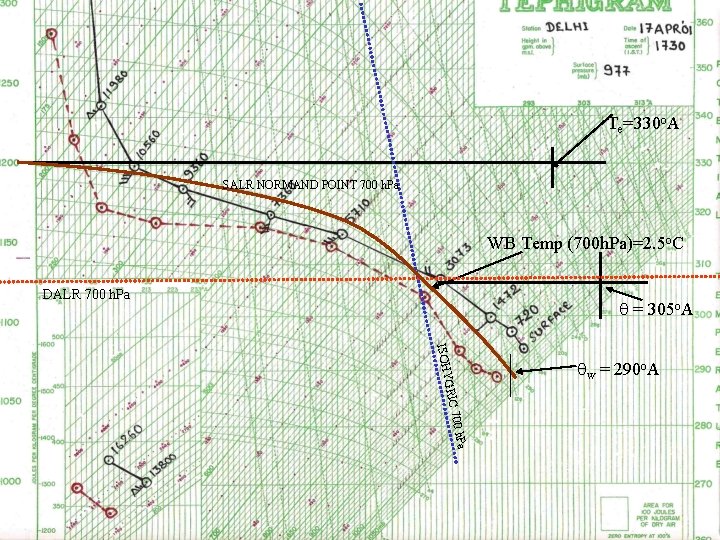
Te=330 o. A SALR NORMAND POINT 700 h. Pa WB Temp (700 h. Pa)=2. 5 o. C DALR 700 h. Pa = 305 o. A 0 h. Pa 70 GRIC ISOHY w = 290 o. A

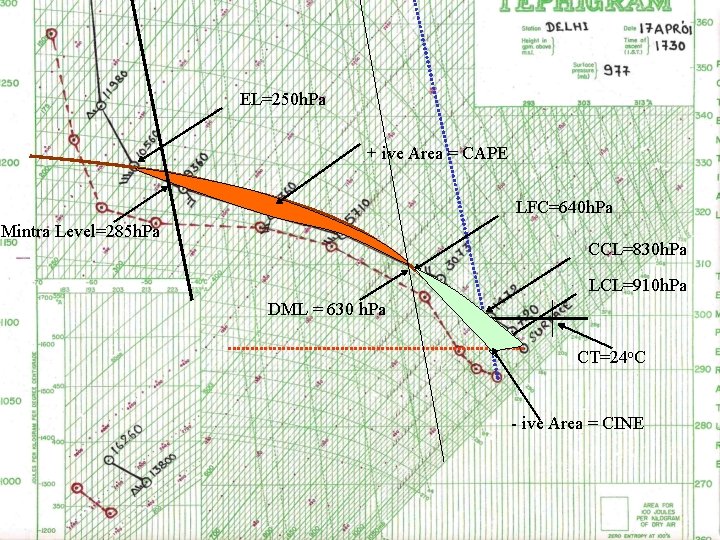
EL=250 h. Pa + ive Area = CAPE LFC=640 h. Pa Mintra Level=285 h. Pa CCL=830 h. Pa LCL=910 h. Pa DML = 630 h. Pa CT=24 o. C - ive Area = CINE

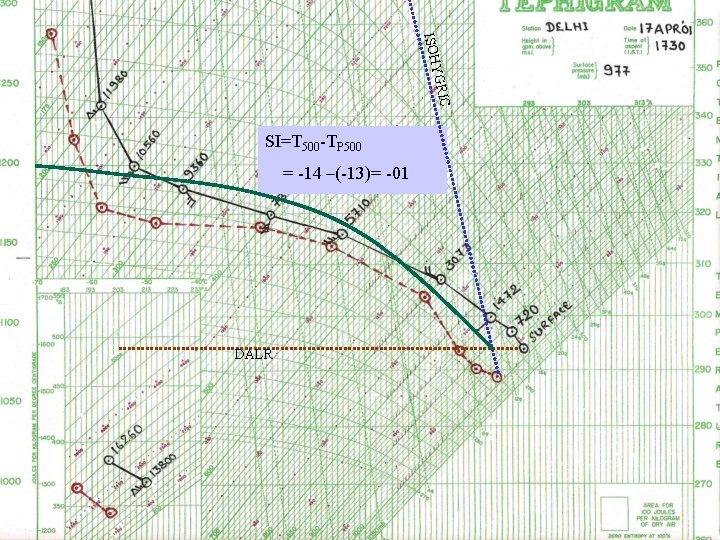
ISOH YGRI C SI=T 500 -TP 500 = -14 –(-13)= -01 DALR

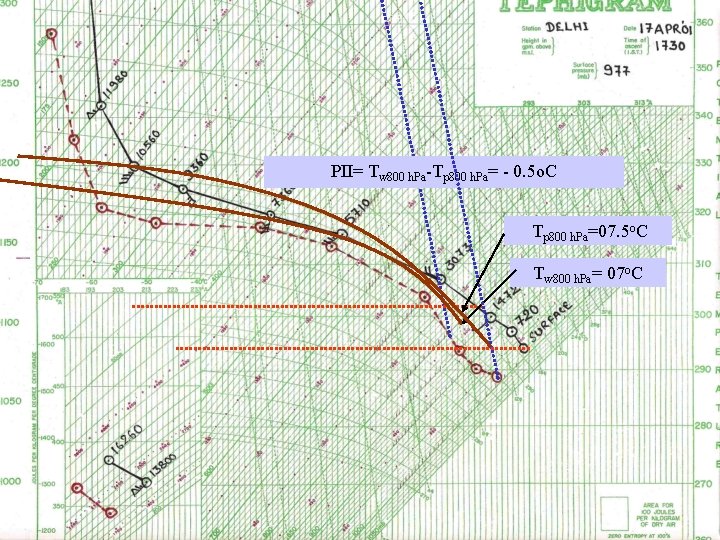
PII= Tw 800 h. Pa-Tp 800 h. Pa= - 0. 5 o. C Tp 800 h. Pa=07. 5 o. C Tw 800 h. Pa= 07 o. C

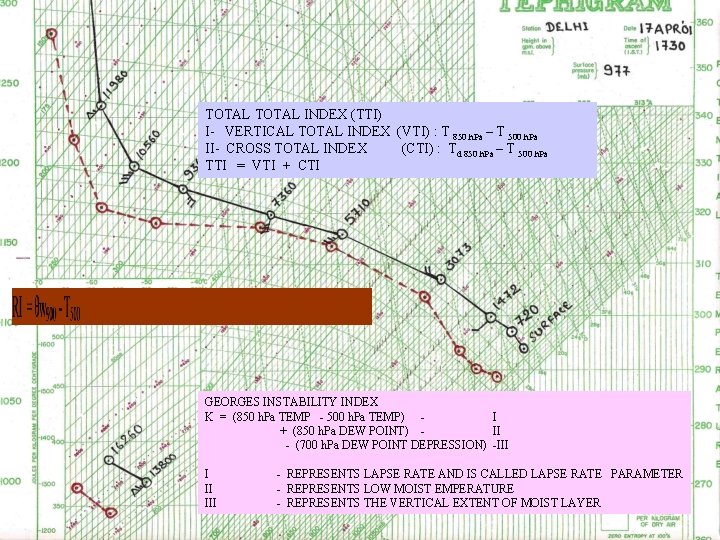
TOTAL INDEX (TTI) I- VERTICAL TOTAL INDEX (VTI) : T 850 h. Pa – T 500 h. Pa II- CROSS TOTAL INDEX (CTI) : Td 850 h. Pa – T 500 h. Pa TTI = VTI + CTI GEORGES INSTABILITY INDEX K = (850 h. Pa TEMP - 500 h. Pa TEMP) I + (850 h. Pa DEW POINT) II - (700 h. Pa DEW POINT DEPRESSION) -III III - REPRESENTS LAPSE RATE AND IS CALLED LAPSE RATE PARAMETER - REPRESENTS LOW MOIST EMPERATURE - REPRESENTS THE VERTICAL EXTENT OF MOIST LAYER

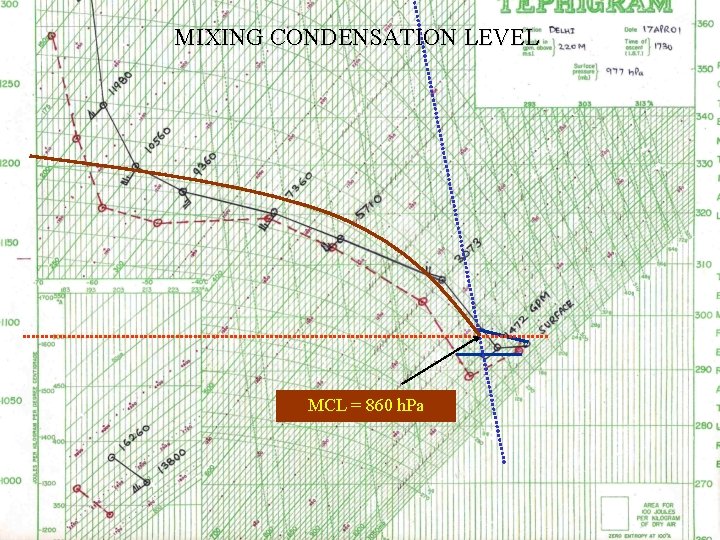
MIXING CONDENSATION LEVEL MCL = 860 h. Pa

CONCLUSION • THE STANDARD ELEMENTS OBSERVED FROM RADIO-SONDE ASCENTS ARE PRESSURE, TEMPERATURE AND HUMIDITY. THESE ELEMENTS CAN BE VERY SIMPLY REPRESENTED ON A TEPHIGRAM. • TWO CURVES ARE USUALLY PLOTTED AND DRAWN ON THE TEPHIGRAM FOR EACH SOUNDINGS. • ONE REPRESENTS THE FREE-AIR TEMPERATURE (T) AND THE OTHER THE DEW-POINT TEMPERATURE (Td). • FROM A PLOTTED TEPHIGRAM CERTAIN METEOROLOGICAL QUANTITIES, SUCH AS THE HUMIDITY PARAMETERS LIKE MIXING RATIO, VAPOUR PRESSURE, RELATIVE HUMIDITY ETC. , AND THERMODYNAMIC PARAMETERS LIKE POTENTIAL TEMPERATURE, EQUIVALENT TEMPERATURES ETC. MAY BE EVALUATED. • BY COMPUTING THE VARIOUS INDICES IT ALSO HELPS IN DETERMINING THE STABILITY OF THE ATMOSPHERE. PREDICTION OF CONVECTIVE PHENOMENON.
 Aerological diagrams
Aerological diagrams Aerological diagram
Aerological diagram Thermodynamic property relations
Thermodynamic property relations Pericyclic
Pericyclic Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Thermodynamic process
Thermodynamic process Thermodynamic
Thermodynamic Formula sheet thermodynamics
Formula sheet thermodynamics Maxwell's relations thermodynamics
Maxwell's relations thermodynamics Vanthoff equation
Vanthoff equation Intensive properties thermodynamics
Intensive properties thermodynamics Thermodynamic control
Thermodynamic control Cp-cv=r/m
Cp-cv=r/m Maxwell thermodynamic relations
Maxwell thermodynamic relations Zeroth law of thermodynamics
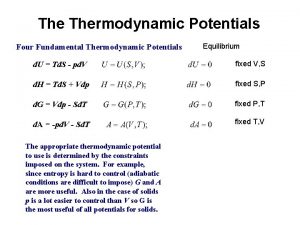
Zeroth law of thermodynamics U=ts-pv
U=ts-pv Bergeron process definition
Bergeron process definition What is thermodynamic equilibrium constant
What is thermodynamic equilibrium constant Second law of thermodynamic
Second law of thermodynamic Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Thorpe ingold effect
Thorpe ingold effect Gibbs free energy equation
Gibbs free energy equation Cv and cp relationship
Cv and cp relationship