STATES OF MATTER PHASE CHANGES Solid a Particles











- Slides: 26

STATES OF MATTER & PHASE CHANGES

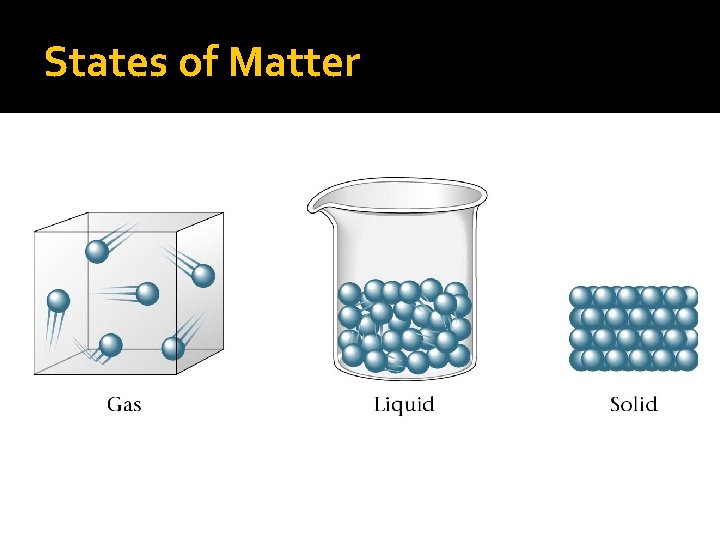
Solid (a) Particles in solid Liquid (b) Particles in liquid Gas (c) Particles in gas

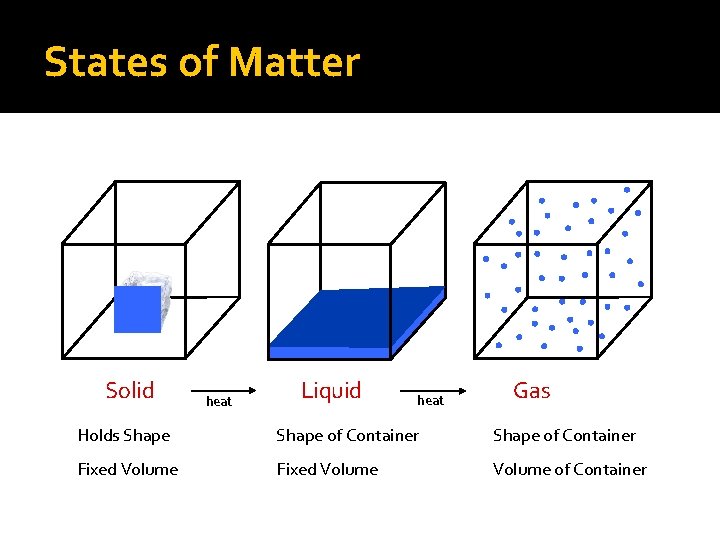
States of Matter Solid heat Liquid heat Gas Holds Shape of Container Fixed Volume of Container

States of Matter Solid heat Liquid heat Gas

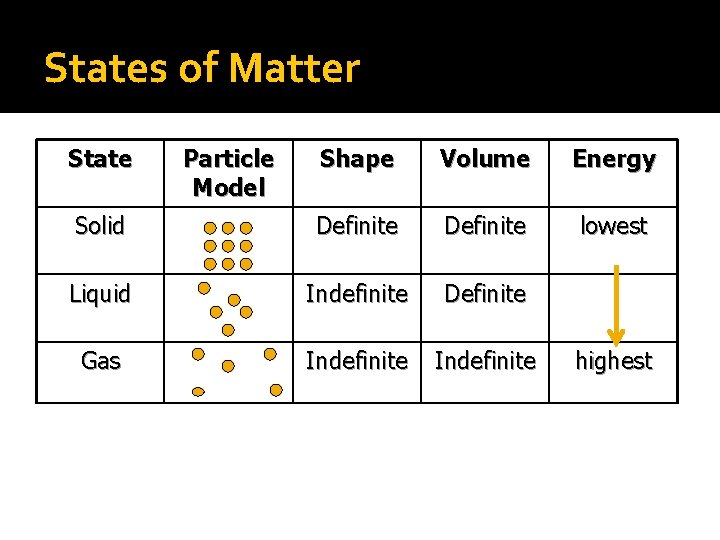
States of Matter State Particle Model Shape Volume Energy Solid Definite lowest Liquid Indefinite Definite Gas Indefinite highest

Characteristics of Solids �Particles are very tightly packed �Definite shape, definite volume �Particles vibrate but cannot move

Characteristics of Liquids �Looser attraction between particles �indefinite shape, definite volume �Particles can tumble over each other �Liquids can flow

Characteristics of Gases �No attraction between particles �indefinite shape, indefinite volume �Particles move rapidly and are in constant motion �Gases can flow

What is the state of oobleck? �Discuss with your group which state of matter oobleck should be classified as. �Cite evidence for your answer! �http: //www. youtube. com/watch? v=y. Hl. Ac. ASs f 6 U

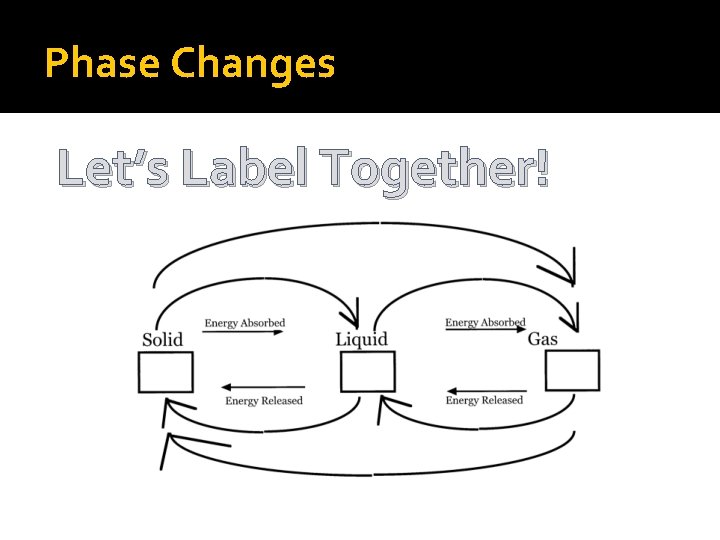
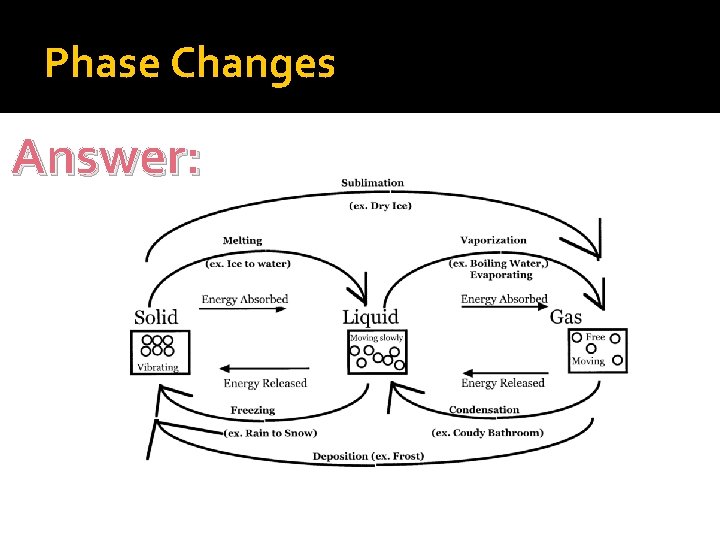
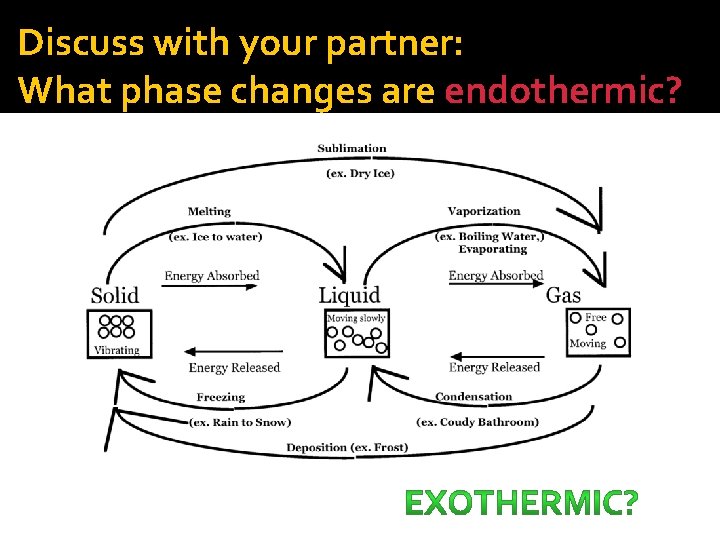
Phase Changes �Melting �Freezing �Vaporization �Condensation �Deposition �Sublimation

Phase Changes Let’s Label Together!

Phase Changes Answer:

Food for Thought �There is a 4 th state of matter known as plasma. �A plasma is an ionized gas �A plasma is a very good conductor of electricity and is affected by magnetic fields. �Examples:

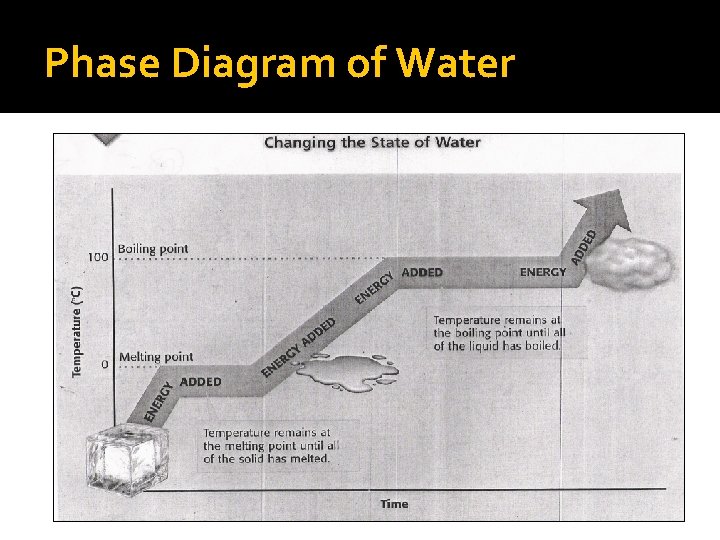
Phase Diagram of Water

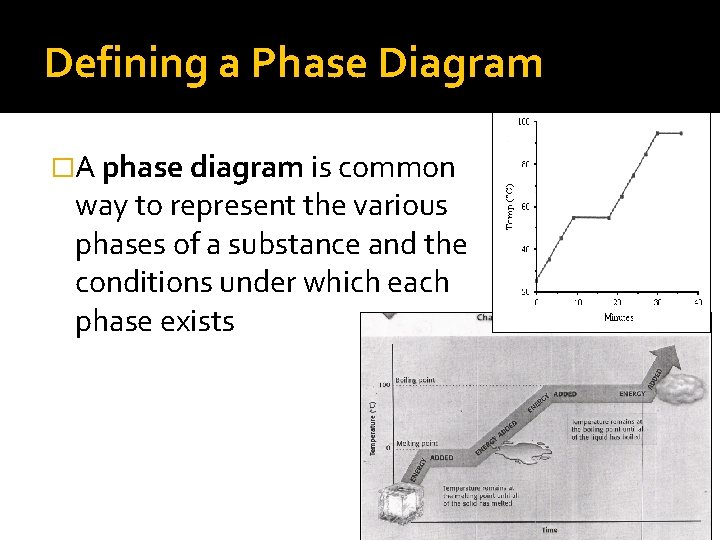
Defining a Phase Diagram �A phase diagram is common way to represent the various phases of a substance and the conditions under which each phase exists

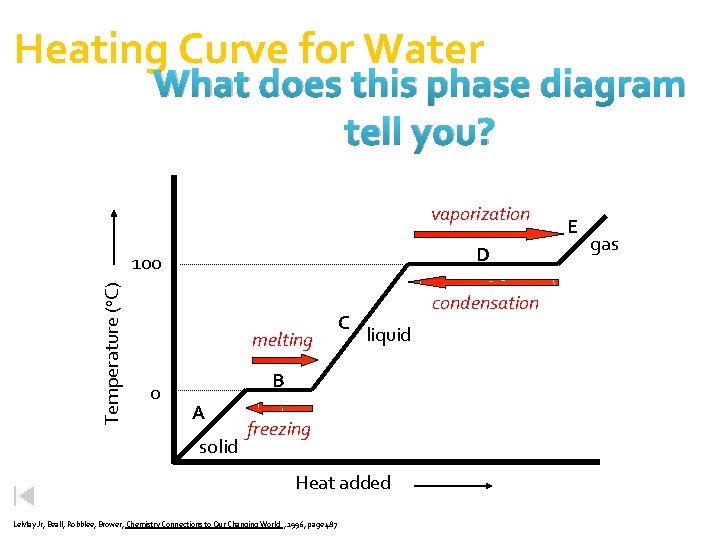
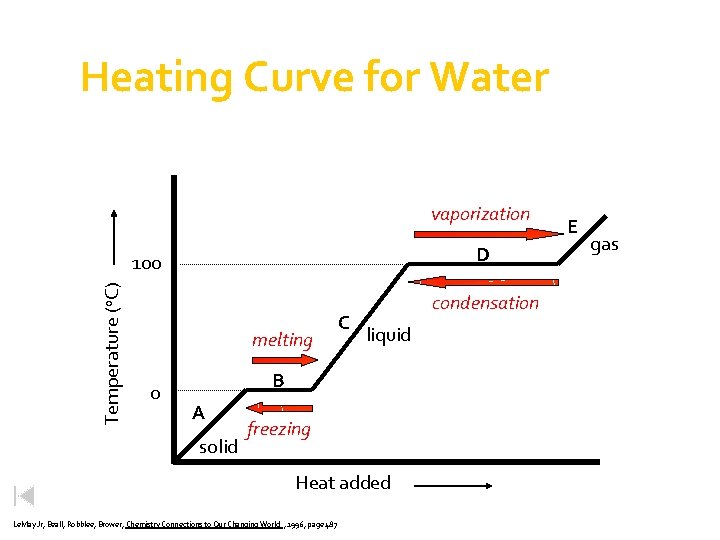
Heating Curve for Water What does this phase diagram tell you? vaporization D Temperature (o. C) 100 melting 0 C condensation liquid B A solid freezing Heat added Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 487 E gas

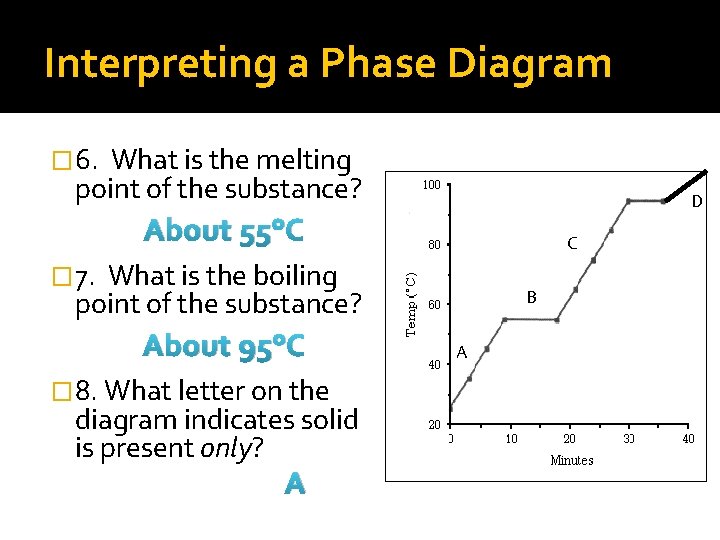
Interpreting a Phase Diagram � 6. What is the melting point of the substance? D About 55°C C � 7. What is the boiling point of the substance? About 95°C � 8. What letter on the diagram indicates solid is present only? A B A

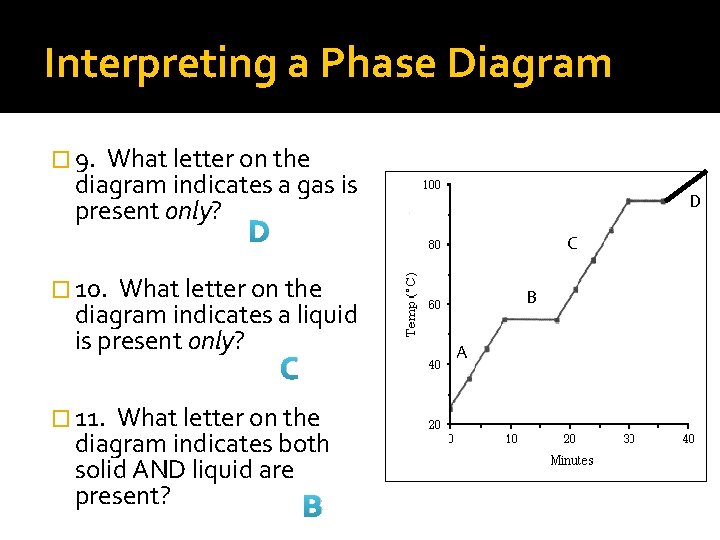
Interpreting a Phase Diagram � 9. What letter on the diagram indicates a gas is present only? D D C � 10. What letter on the diagram indicates a liquid is present only? C � 11. What letter on the diagram indicates both solid AND liquid are present? B B A

Questions about Phase Change Lab? �Analysis Questions �Claims and Evidence �Reflection Questions �LAB REPORT DUE THURSDAY, APRIL 4 th.

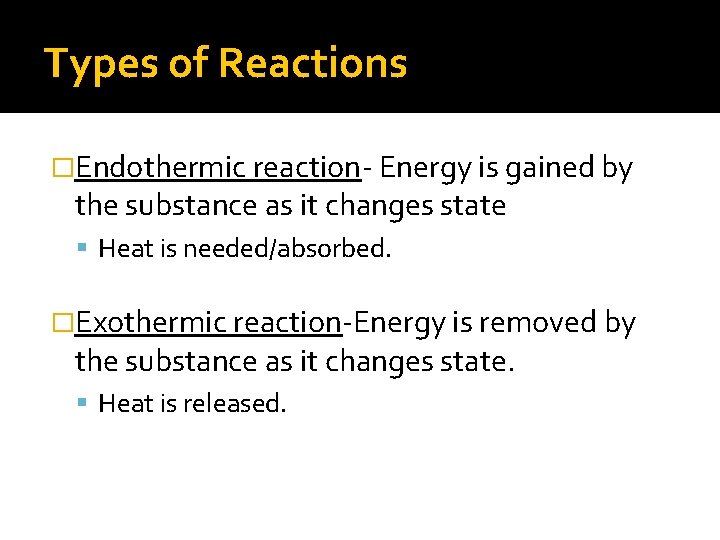
Types of Reactions �Endothermic reaction- Energy is gained by the substance as it changes state Heat is needed/absorbed. �Exothermic reaction-Energy is removed by the substance as it changes state. Heat is released.

Discuss with your partner: What phase changes are endothermic?

Phase Changes - Melting �Melting is when a substance changes from a solid to a liquid. �Do particles gain or lose speed? The particles increase their speed causing them to break apart from each other. �Endothermic or Exothermic Reaction? �Examples-melting snow or ice

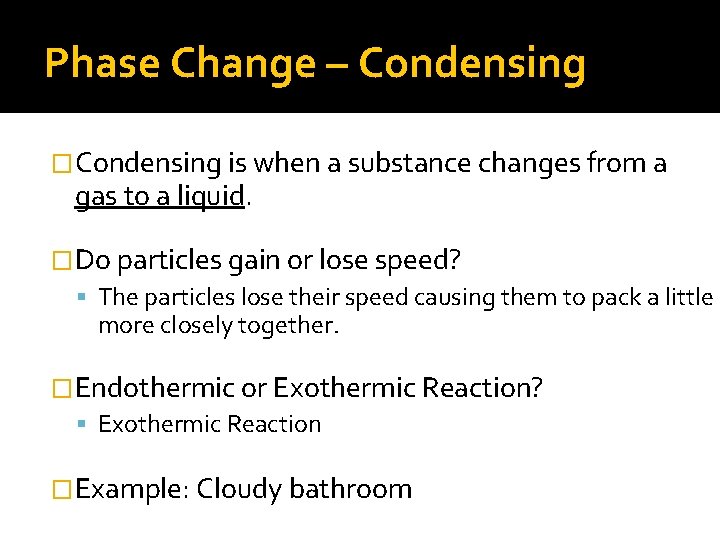
Phase Change – Condensing �Condensing is when a substance changes from a gas to a liquid. �Do particles gain or lose speed? The particles lose their speed causing them to pack a little more closely together. �Endothermic or Exothermic Reaction? Exothermic Reaction �Example: Cloudy bathroom

Let’s Change some Phases by… �MAKING ICE CREAM!

Take a Look at Your Lab �CHANGE PROCEDURE #5 TO SAY ½ CUP OF ROCK SALT

Heating Curve for Water vaporization D Temperature (o. C) 100 melting 0 C condensation liquid B A solid freezing Heat added Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 487 E gas