12242021 Radiation and the Universe AQA Electromagnetic Radiation






- Slides: 31

12/24/2021 Radiation and the Universe (AQA)

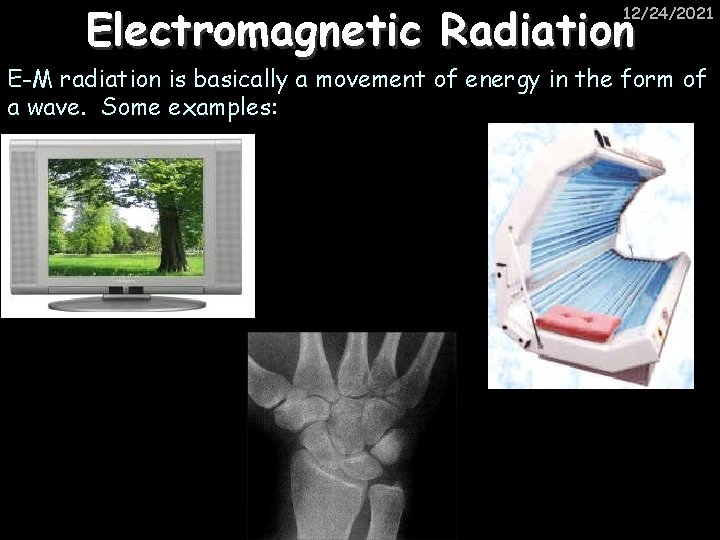
Electromagnetic Radiation 12/24/2021 E-M radiation is basically a movement of energy in the form of a wave. Some examples:

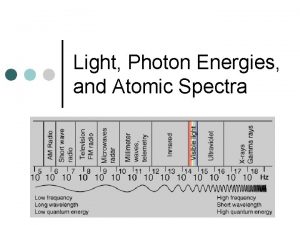
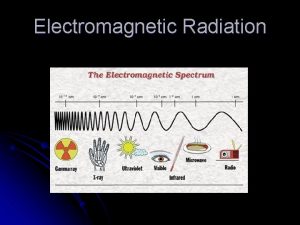
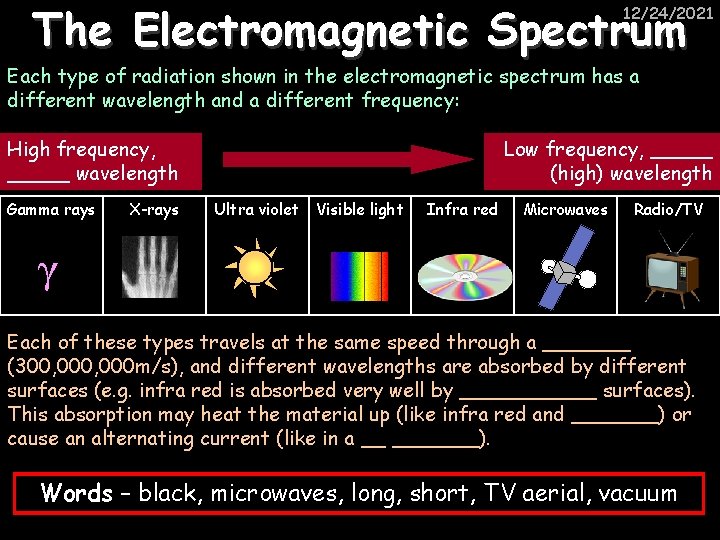
The Electromagnetic Spectrum 12/24/2021 Each type of radiation shown in the electromagnetic spectrum has a different wavelength and a different frequency: High frequency, _____ wavelength Gamma rays X-rays Low frequency, _____ (high) wavelength Ultra violet Visible light Infra red Microwaves Radio/TV γ Each of these types travels at the same speed through a _______ (300, 000 m/s), and different wavelengths are absorbed by different surfaces (e. g. infra red is absorbed very well by ______ surfaces). This absorption may heat the material up (like infra red and _______) or cause an alternating current (like in a __ _______). Words – black, microwaves, long, short, TV aerial, vacuum

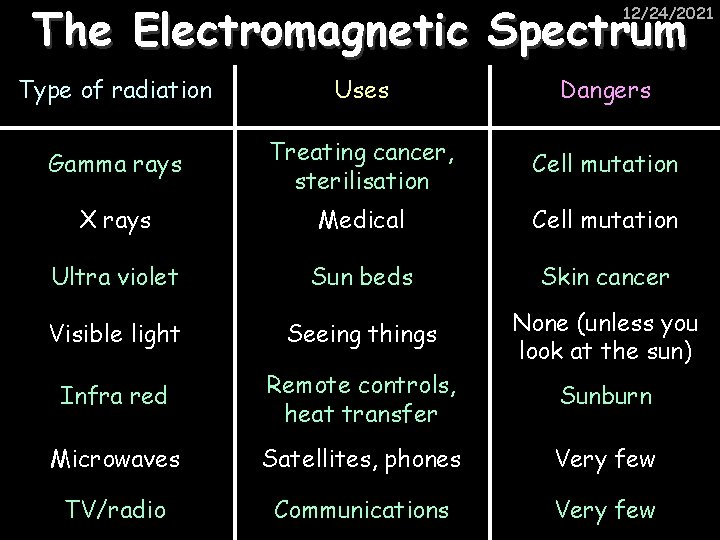
The Electromagnetic Spectrum 12/24/2021 Type of radiation Uses Dangers Gamma rays Treating cancer, sterilisation Cell mutation X rays Medical Cell mutation Ultra violet Sun beds Skin cancer Visible light Seeing things None (unless you look at the sun) Infra red Remote controls, heat transfer Sunburn Microwaves Satellites, phones Very few TV/radio Communications Very few

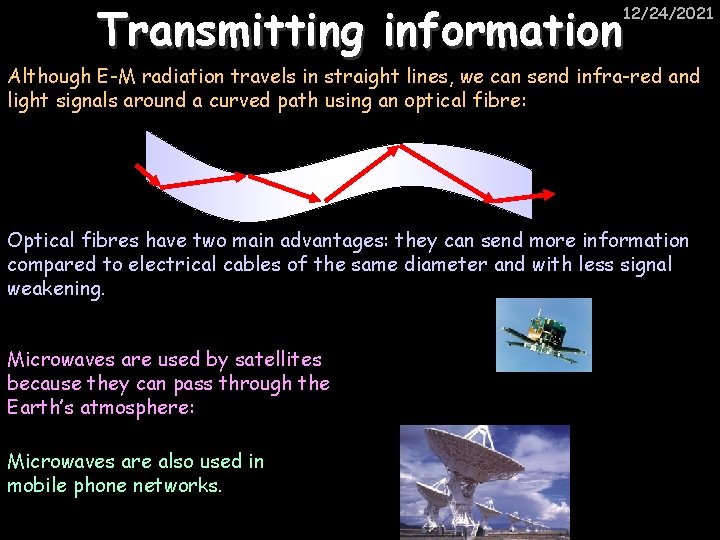
Transmitting information 12/24/2021 Although E-M radiation travels in straight lines, we can send infra-red and light signals around a curved path using an optical fibre: Optical fibres have two main advantages: they can send more information compared to electrical cables of the same diameter and with less signal weakening. Microwaves are used by satellites because they can pass through the Earth’s atmosphere: Microwaves are also used in mobile phone networks.

The Wave Equation 12/24/2021 All E-M waves obey the Wave Equation: Wave speed (v) = frequency (f) x wavelength ( ) in m/s in Hz in m V f

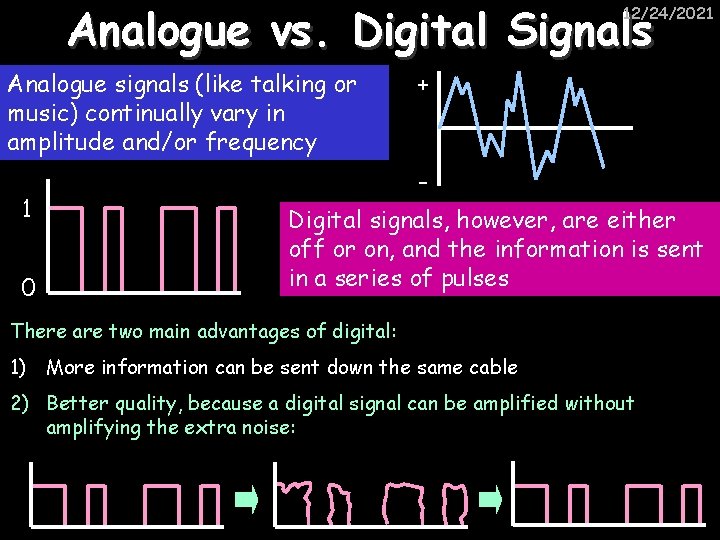
Analogue vs. Digital Signals 12/24/2021 Analogue signals (like talking or music) continually vary in amplitude and/or frequency 1 0 + Digital signals, however, are either off or on, and the information is sent in a series of pulses There are two main advantages of digital: 1) More information can be sent down the same cable 2) Better quality, because a digital signal can be amplified without amplifying the extra noise:

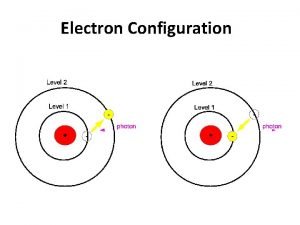
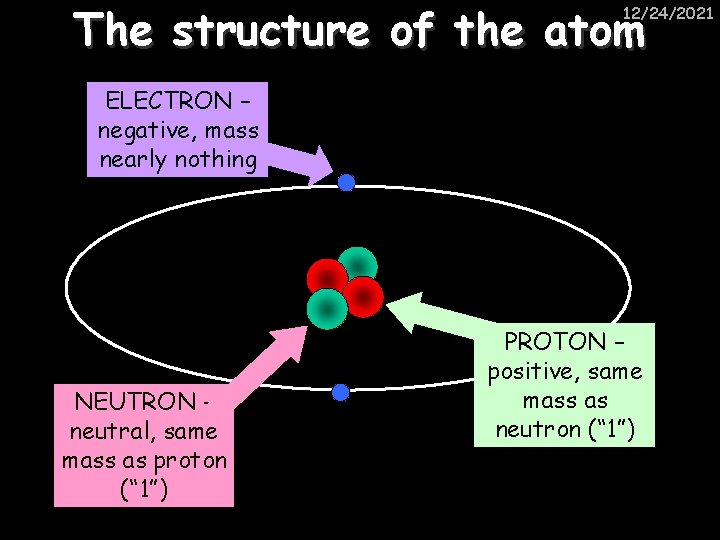
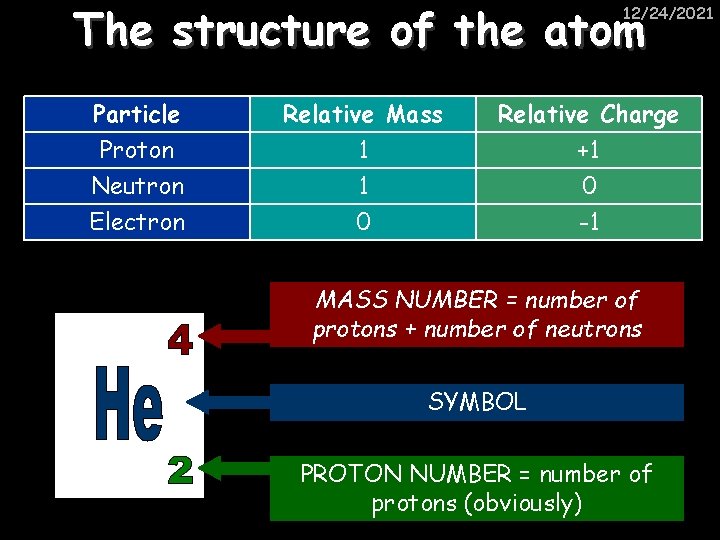
The structure of the atom 12/24/2021 ELECTRON – negative, mass nearly nothing NEUTRON – neutral, same mass as proton (“ 1”) PROTON – positive, same mass as neutron (“ 1”)

The structure of the atom 12/24/2021 Particle Proton Neutron Electron Relative Mass 1 1 0 Relative Charge +1 0 -1 MASS NUMBER = number of protons + number of neutrons SYMBOL PROTON NUMBER = number of protons (obviously)

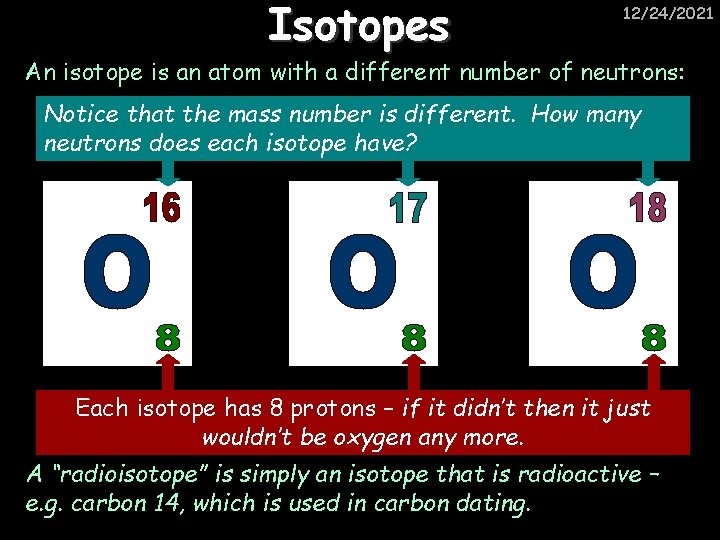
Isotopes 12/24/2021 An isotope is an atom with a different number of neutrons: Notice that the mass number is different. How many neutrons does each isotope have? Each isotope has 8 protons – if it didn’t then it just wouldn’t be oxygen any more. A “radioisotope” is simply an isotope that is radioactive – e. g. carbon 14, which is used in carbon dating.

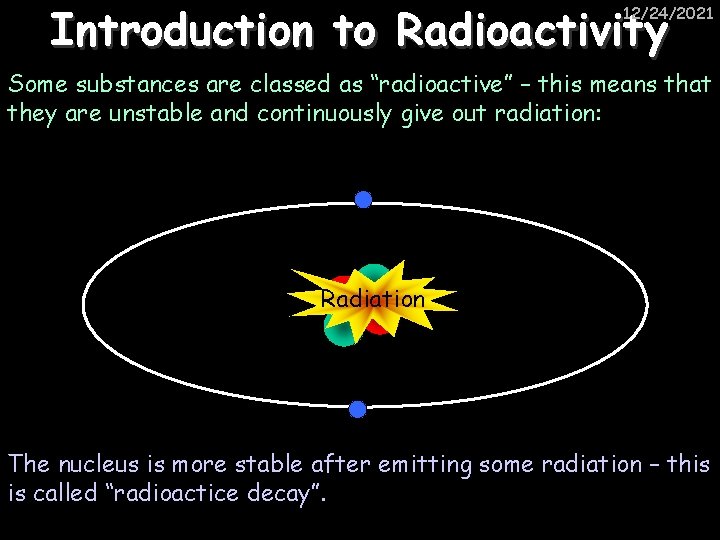
Introduction to Radioactivity 12/24/2021 Some substances are classed as “radioactive” – this means that they are unstable and continuously give out radiation: Radiation The nucleus is more stable after emitting some radiation – this is called “radioactice decay”.

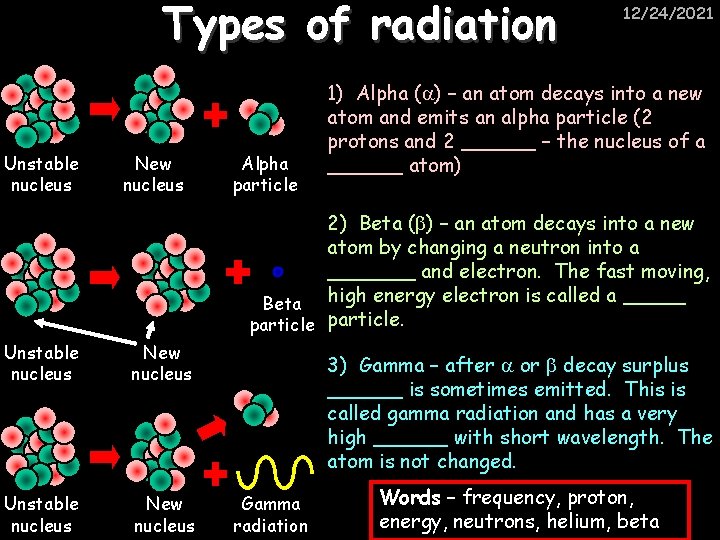
Types of radiation Unstable nucleus New nucleus Alpha particle 12/24/2021 1) Alpha ( ) – an atom decays into a new atom and emits an alpha particle (2 protons and 2 ______ – the nucleus of a ______ atom) 2) Beta ( ) – an atom decays into a new atom by changing a neutron into a _______ and electron. The fast moving, Beta high energy electron is called a _____ particle. Unstable nucleus New nucleus 3) Gamma – after or decay surplus ______ is sometimes emitted. This is called gamma radiation and has a very high ______ with short wavelength. The atom is not changed. Gamma radiation Words – frequency, proton, energy, neutrons, helium, beta

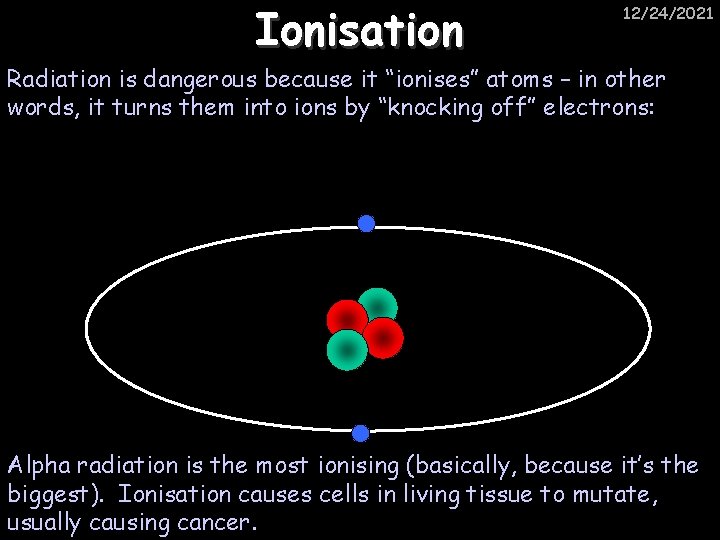
Ionisation 12/24/2021 Radiation is dangerous because it “ionises” atoms – in other words, it turns them into ions by “knocking off” electrons: Alpha radiation is the most ionising (basically, because it’s the biggest). Ionisation causes cells in living tissue to mutate, usually causing cancer.

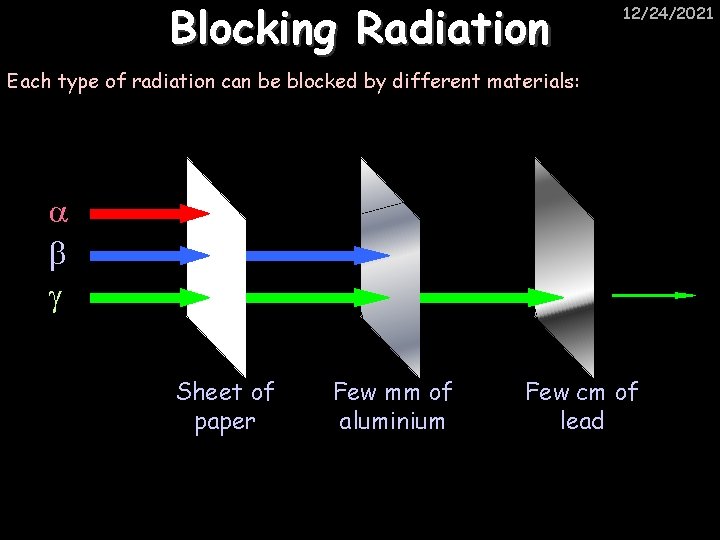
Blocking Radiation 12/24/2021 Each type of radiation can be blocked by different materials: Sheet of paper Few mm of aluminium Few cm of lead

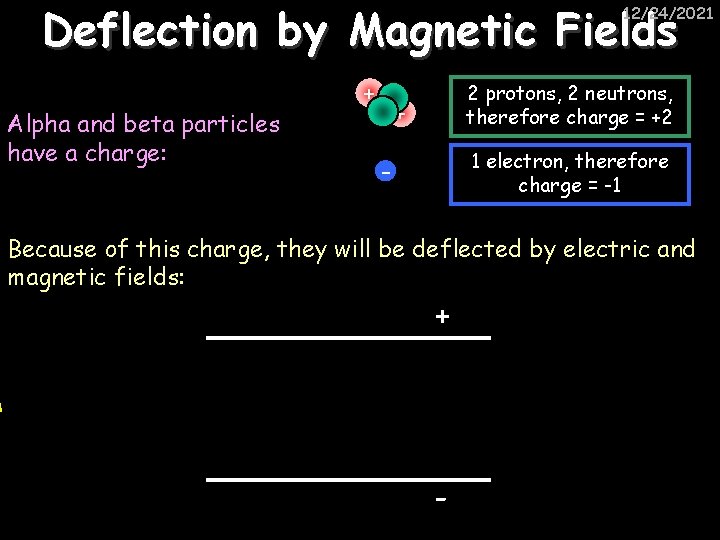
Deflection by Magnetic Fields 12/24/2021 Alpha and beta particles have a charge: + 2 protons, 2 neutrons, therefore charge = +2 + 1 electron, therefore charge = -1 - Because of this charge, they will be deflected by electric and magnetic fields: + -

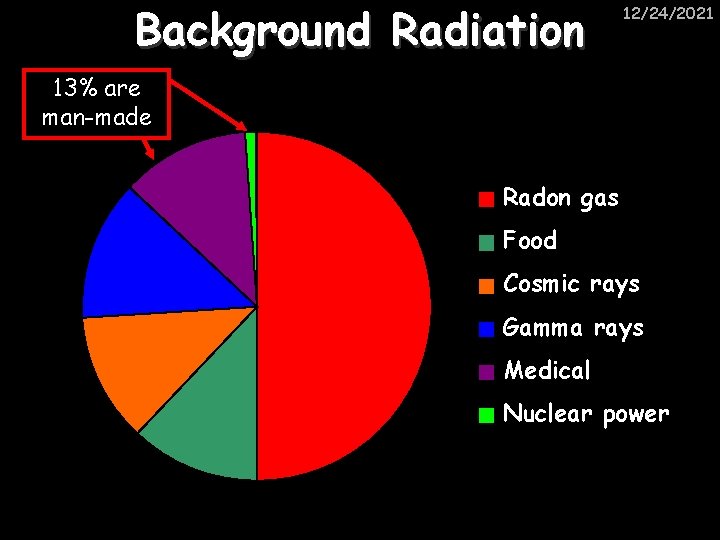
Background Radiation 12/24/2021 13% are man-made Radon gas Food Cosmic rays Gamma rays Medical Nuclear power

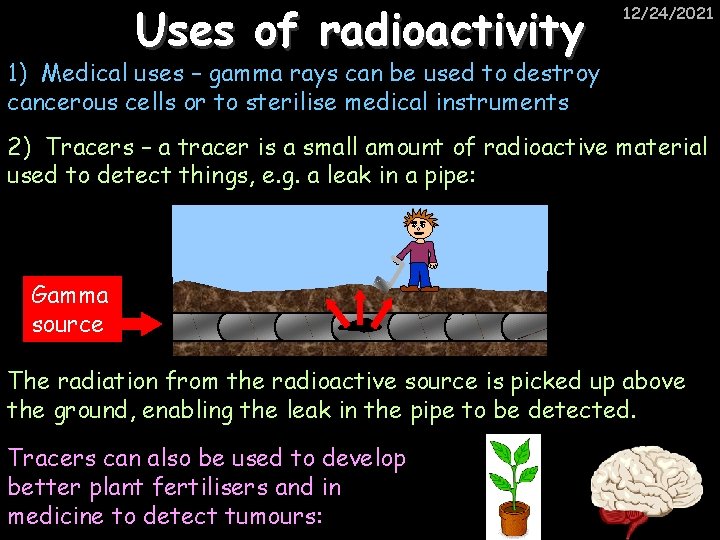
Uses of radioactivity 12/24/2021 1) Medical uses – gamma rays can be used to destroy cancerous cells or to sterilise medical instruments 2) Tracers – a tracer is a small amount of radioactive material used to detect things, e. g. a leak in a pipe: Gamma source The radiation from the radioactive source is picked up above the ground, enabling the leak in the pipe to be detected. Tracers can also be used to develop better plant fertilisers and in medicine to detect tumours:

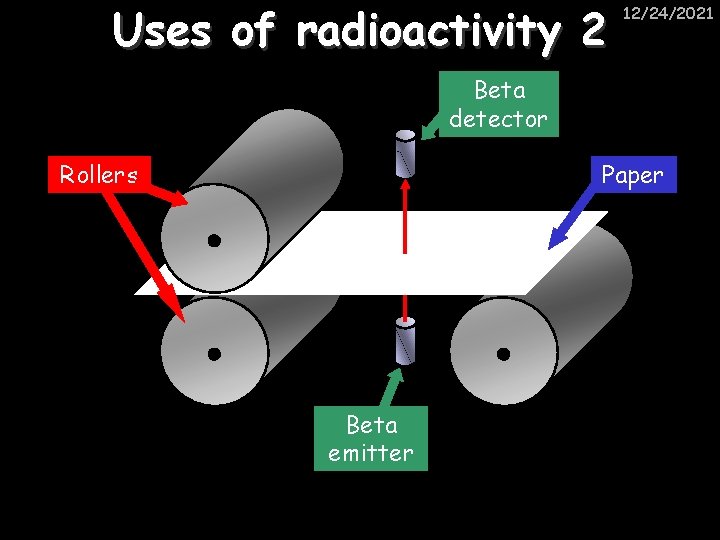
Uses of radioactivity 2 12/24/2021 Beta detector Rollers Paper Beta emitter

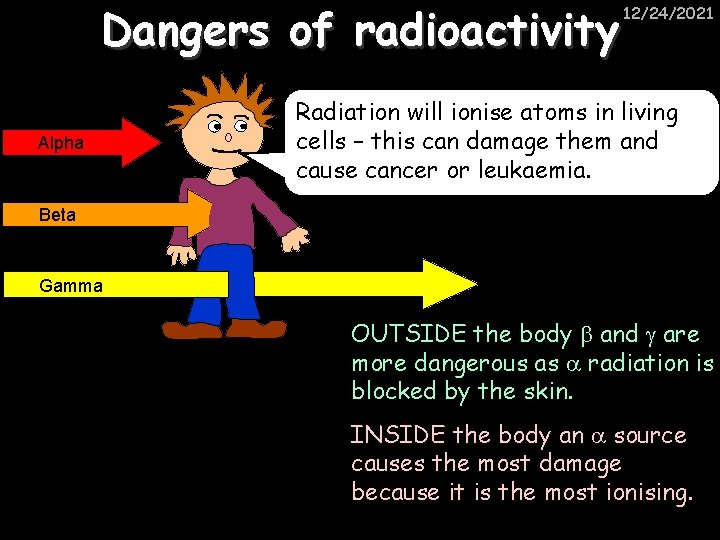
Dangers of radioactivity Alpha 12/24/2021 Radiation will ionise atoms in living cells – this can damage them and cause cancer or leukaemia. Beta Gamma OUTSIDE the body and are more dangerous as radiation is blocked by the skin. INSIDE the body an source causes the most damage because it is the most ionising.

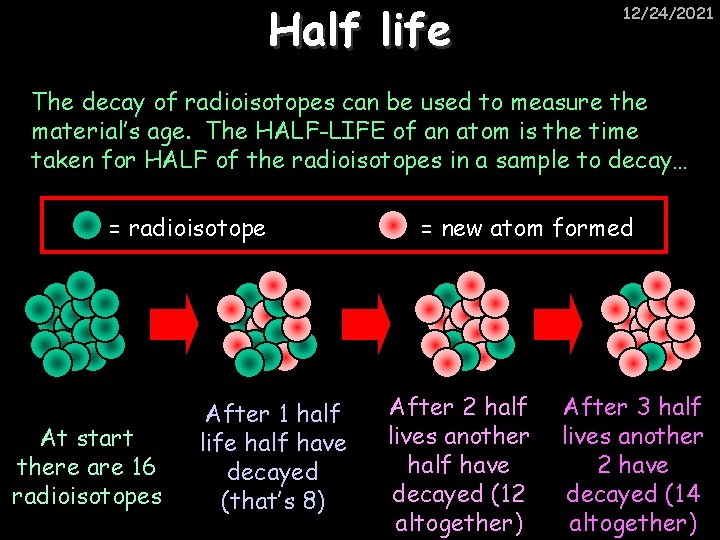
Half life 12/24/2021 The decay of radioisotopes can be used to measure the material’s age. The HALF-LIFE of an atom is the time taken for HALF of the radioisotopes in a sample to decay… = radioisotope At start there are 16 radioisotopes After 1 half life half have decayed (that’s 8) = new atom formed After 2 half lives another half have decayed (12 altogether) After 3 half lives another 2 have decayed (14 altogether)

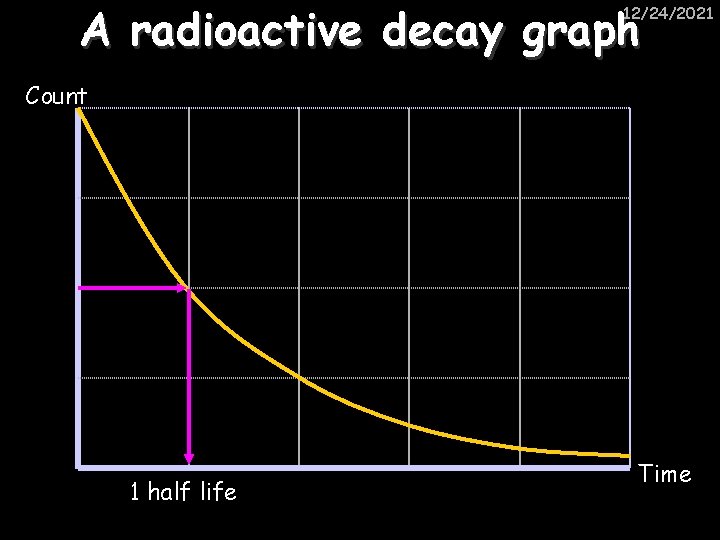
A radioactive decay graph 12/24/2021 Count 1 half life Time

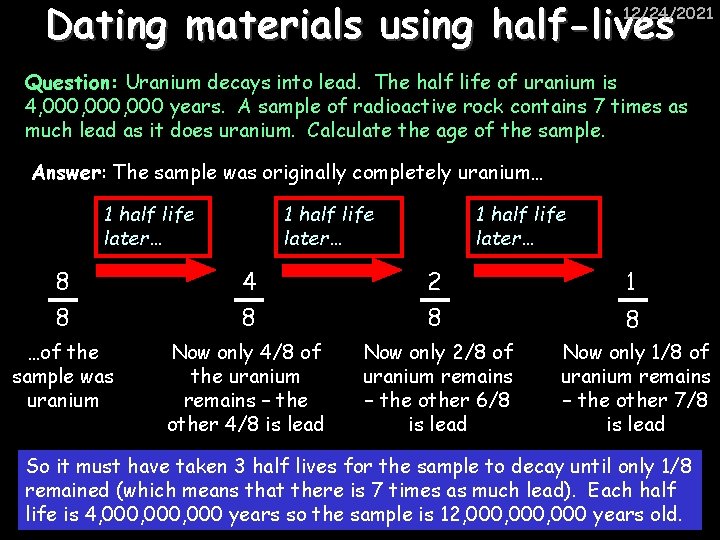
Dating materials using half-lives 12/24/2021 Question: Uranium decays into lead. The half life of uranium is 4, 000, 000 years. A sample of radioactive rock contains 7 times as much lead as it does uranium. Calculate the age of the sample. Answer: The sample was originally completely uranium… 1 half life later… 8 8 4 8 2 8 1 …of the sample was uranium Now only 4/8 of the uranium remains – the other 4/8 is lead Now only 2/8 of uranium remains – the other 6/8 is lead Now only 1/8 of uranium remains – the other 7/8 is lead 8 So it must have taken 3 half lives for the sample to decay until only 1/8 remained (which means that there is 7 times as much lead). Each half life is 4, 000, 000 years so the sample is 12, 000, 000 years old.

An exam question… 12/24/2021 Potassium decays into argon. The half life of potassium is 1. 3 billion years. A sample of rock from Mars is found to contain three argon atoms for every atom of potassium. How old is the rock? (3 marks) The rock must be 2 half lives old – 2. 6 billion years

12/24/2021 Evidence about the origins of the universe…

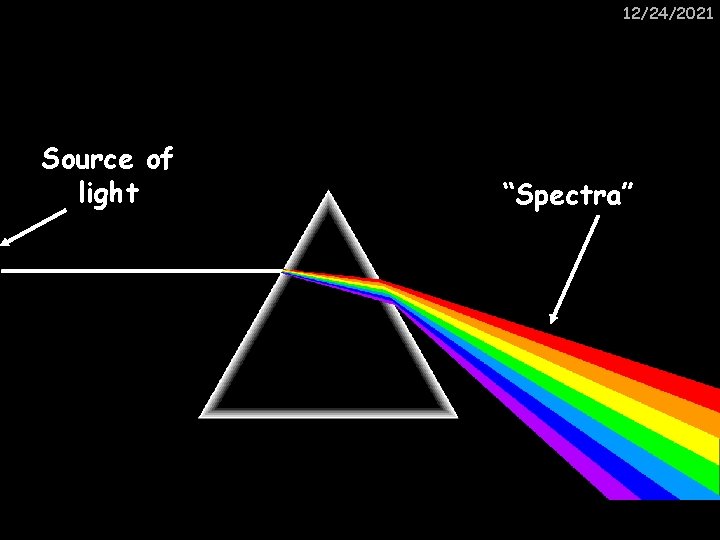
12/24/2021 Source of light “Spectra”

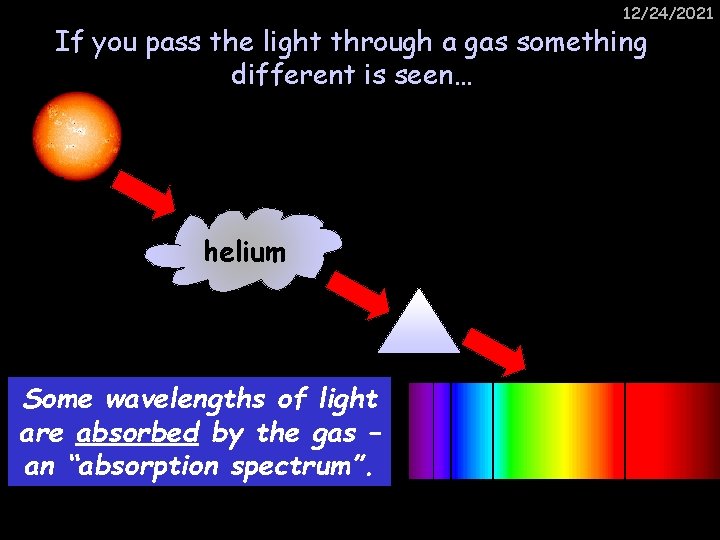
12/24/2021 If you pass the light through a gas something different is seen… helium Some wavelengths of light are absorbed by the gas – an “absorption spectrum”.

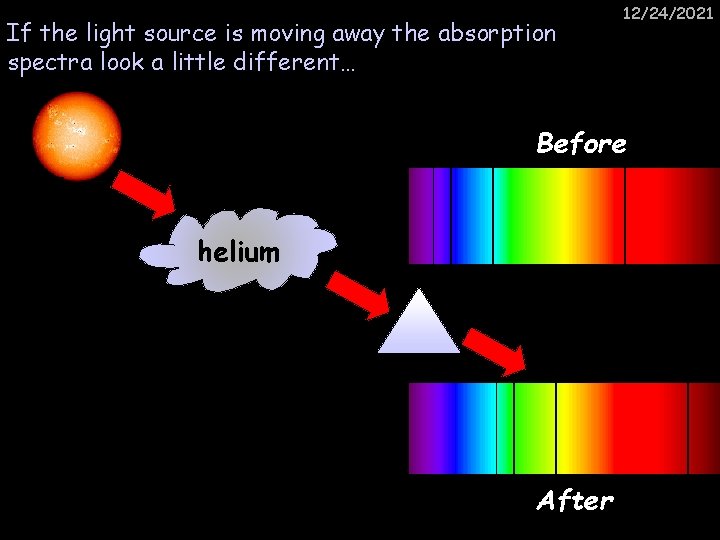
If the light source is moving away the absorption spectra look a little different… 12/24/2021 Before helium After

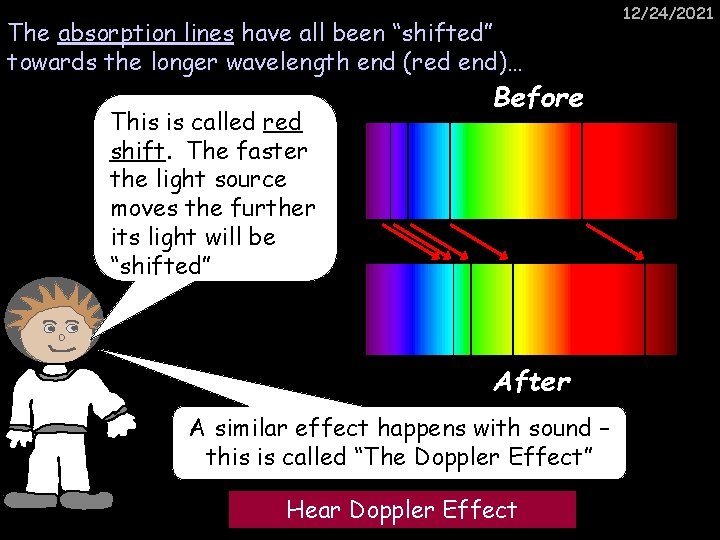
The absorption lines have all been “shifted” towards the longer wavelength end (red end)… This is called red shift. The faster the light source moves the further its light will be “shifted” Before After A similar effect happens with sound – this is called “The Doppler Effect” Hear Doppler Effect 12/24/2021

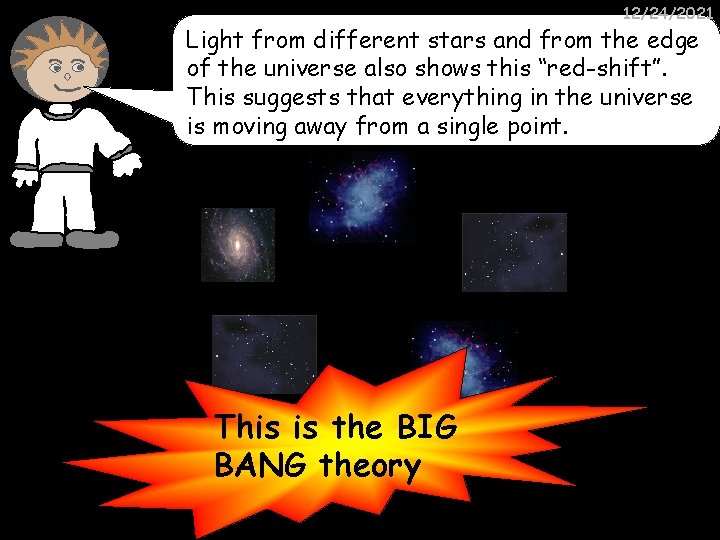
12/24/2021 Light from different stars and from the edge of the universe also shows this “red-shift”. This suggests that everything in the universe is moving away from a single point. This is the BIG BANG theory

Red shift summary 12/24/2021 Light from other galaxies has a longer _____ than expected. This shows that these galaxies are moving ____ from us very quickly. This effect is seen to a greater extent in galaxies that are _______ away from us. This indicates that the further away the galaxy is, the ______ it is moving. This evidence seems to suggest that everything in the universe is moving away from a single point, and that this process started around 15 _____ years ago. This is the ________ Theory. Words to use – faster, away, big bang, billion, wavelength, further

Observing the Universe 12/24/2021 Consider different types of telescope: Ground-based telescopes Space-based telescopes What are the advantages and disadvantages of each?
 Intensity em wave
Intensity em wave Facts about electromagnetic radiation
Facts about electromagnetic radiation Electromagnetic wavelength formula
Electromagnetic wavelength formula Types of radiation in the electromagnetic spectrum
Types of radiation in the electromagnetic spectrum Intensity waves
Intensity waves Which telescope detects invisible electromagnetic radiation
Which telescope detects invisible electromagnetic radiation Electromagnetic waves are transverse waves true or false
Electromagnetic waves are transverse waves true or false Frequency spectrum
Frequency spectrum When electromagnetic radiation of wavelength 300
When electromagnetic radiation of wavelength 300 Em wave spectrum
Em wave spectrum Digital illuminate aqa food preparation and nutrition
Digital illuminate aqa food preparation and nutrition Aqa a level art and design
Aqa a level art and design Aqa fine art gcse
Aqa fine art gcse Aqa design and technology past papers
Aqa design and technology past papers Art and design a level aqa
Art and design a level aqa Food nea
Food nea Ozymandias power and conflict
Ozymandias power and conflict Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan