Electromagnetic Spectrum Electromagnetic Radiation Electromagnetic radiation a form









- Slides: 12

Electromagnetic Spectrum

Electromagnetic Radiation • Electromagnetic radiation – a form of energy that exhibits wavelike behavior as it travels through space • Visible light • Microwaves • X-rays • Radio waves

Waves • Wavelength (λ) – shortest distance between equivalent points in a continuous wave • m, cm, nm • Frequency (ν) – number of waves that pass a given point per second • 1/s = Hz = s-1 • Amplitude – wave’s height from the origin to a crest or trough • All waves travel at the speed of light in a vacuum (2. 998 x 108 m/s)


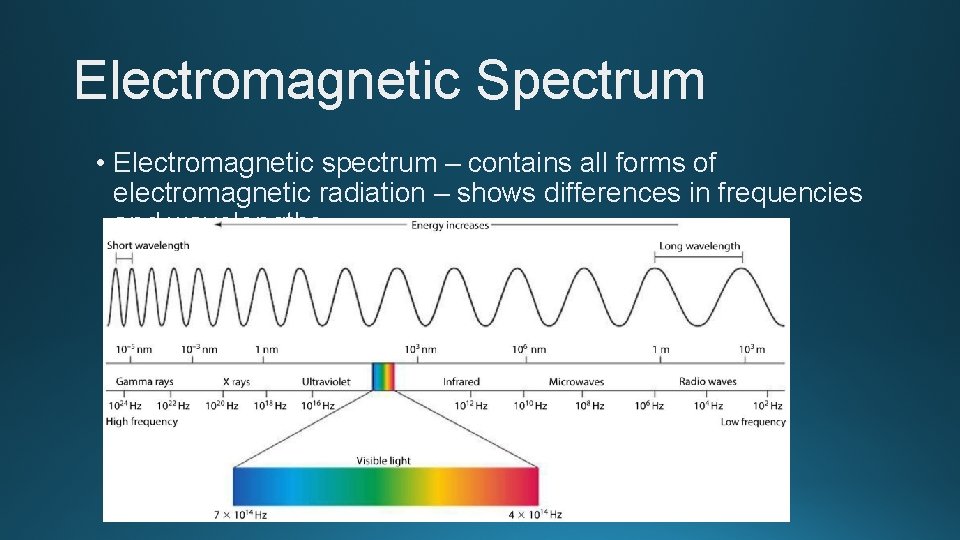
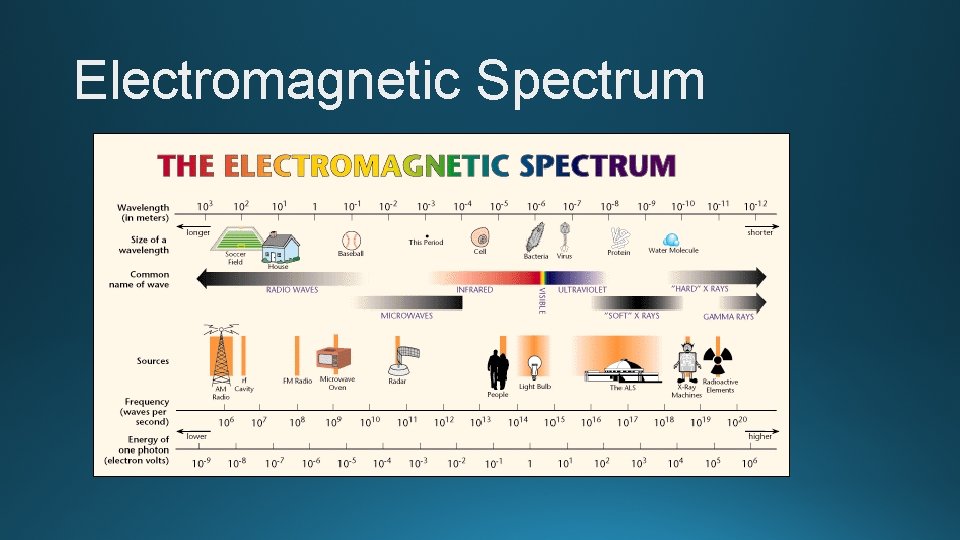
Electromagnetic Spectrum • Electromagnetic spectrum – contains all forms of electromagnetic radiation – shows differences in frequencies and wavelengths

Electromagnetic Spectrum

Wave Calculations • c=λν • C = speed of light (2. 998 x 108 m/s) • λ = wavelength (m) • ν = frequency (Hz; 1/s)

Wave Calculations • Microwaves are used to cook food and transmit information. What is the wavelength of a microwave that has a frequency of 3. 44 x 109 Hz? • Given: • ν = 3. 44 x 109 Hz • c = 2. 998 x 108 m/s Find: λ (=x) • c=λν • 2. 998 x 108 m/s = (x) (3. 44 x 109 Hz) • X = 8. 72 x 10 -2 m

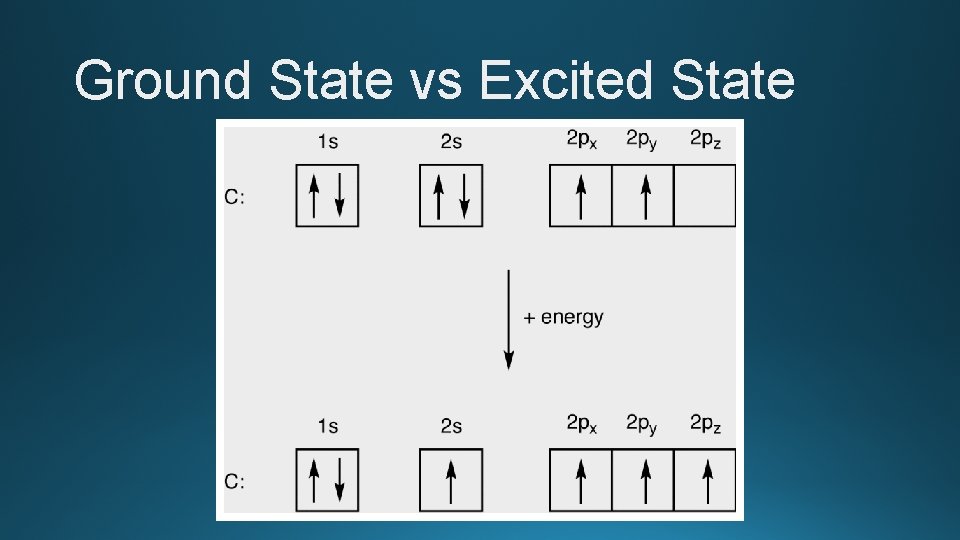
Energy • As electrons absorb energy, they move to higher energy orbits • Excited state electron configuration • 2 s �� 2 p • They will naturally fall back down to their ground state and release the energy gained as a photon of light • Ground state electron configuration • 2 p �� 2 s

Ground State vs Excited State

Energy Calculations • E=hν • E = energy absorbed/emitted • h = Planck’s constant: 6. 626 x 10 -34 J∙sec • ν = frequency (Hz; 1/s) • Remember: c = λ ν • So E = h c λ

Energy Calculations • An electron is excited to the 3 s sublevel from the 2 s sublevel. As the electron falls back down to its ground state, it releases yellow light with a wavelength of 585 nm. What amount of energy did the photon contain? • • Given: λ = 585 nm h = 6. 626 x 10 -34 J∙sec c = 2. 998 x 108 m/s Find: E • E=hc λ • E = (6. 626 x 10 -34 J∙sec) (2. 998 x 108 m/s) 5. 85 x 10 -7 m = 3. 40 x 10 -19 J