1 Razi University KermanshahIran Physical Organic Chemistry Part1















- Slides: 23

1

Razi University Kermanshah-Iran Physical Organic Chemistry Part-1 (Sessions 1 and 2) (For M. S. Students of Organic Chemistry) Prof. Avat Arman Taherpour Department of Organic Chemistry, Faculty of Chemistry, Razi University, P. O. Box: 67149 -67346, Kermanshah, Iran 1398 -99 -2 2

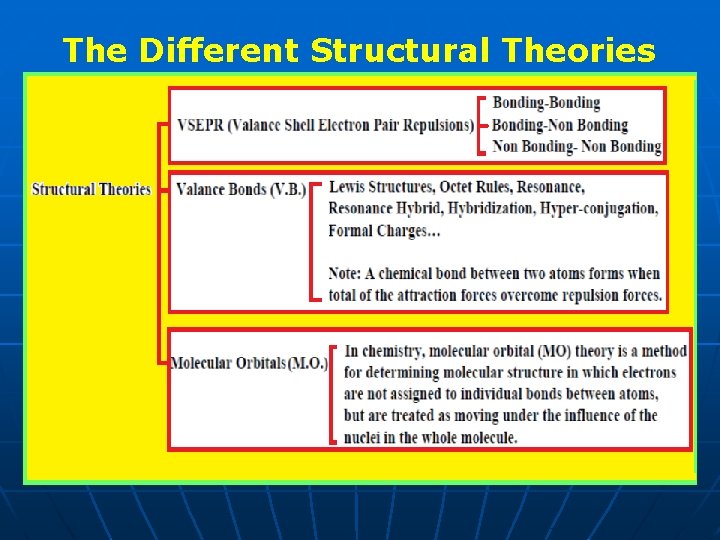
The Different Structural Theories

4

5

6

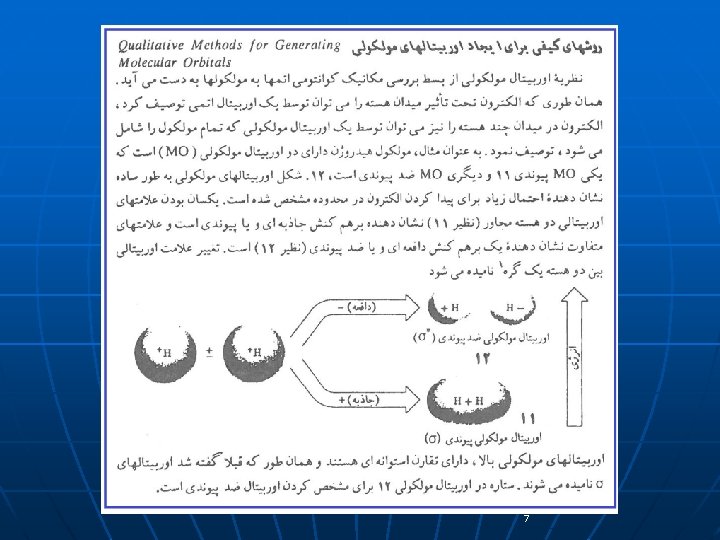
7

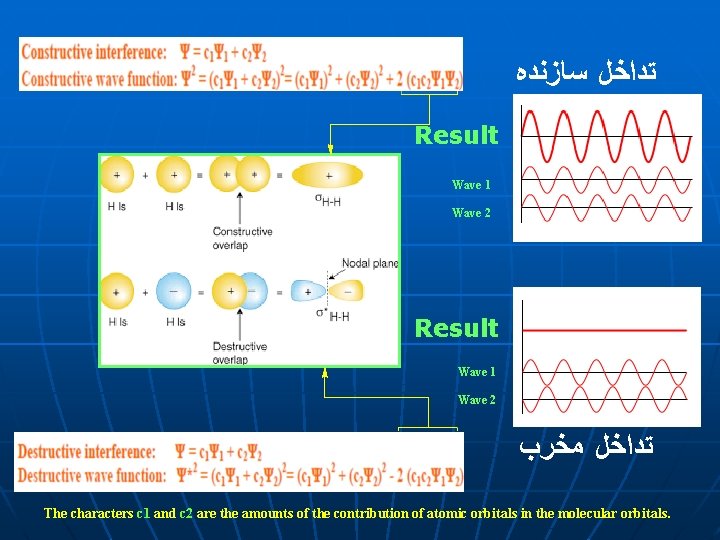
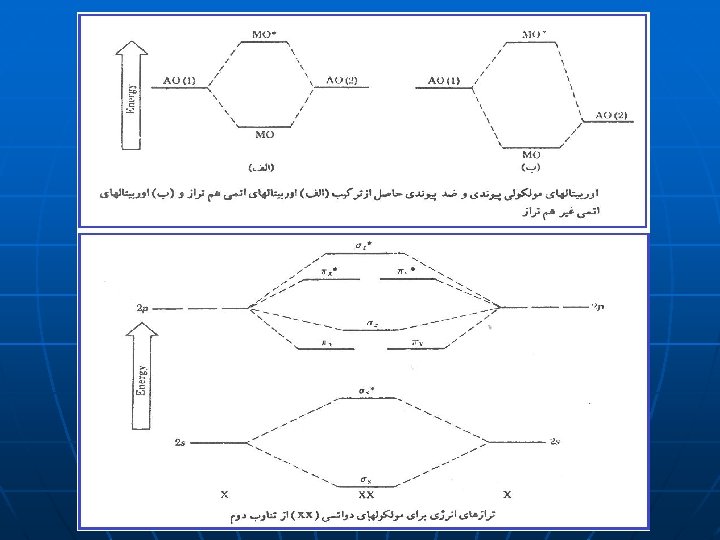
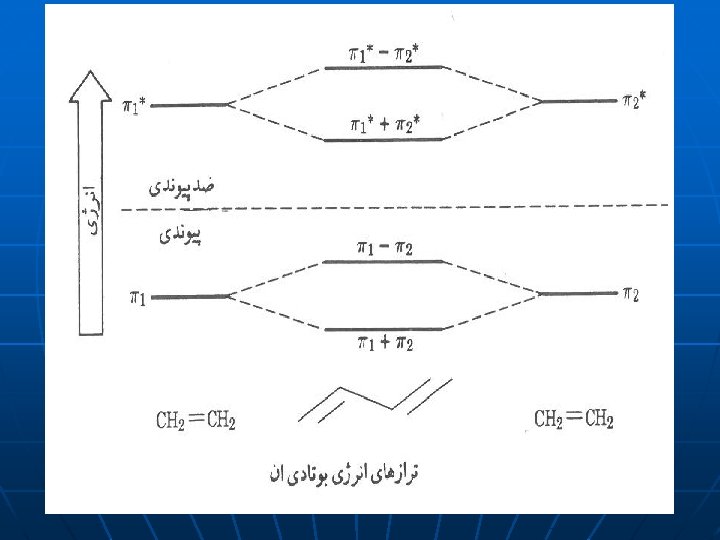
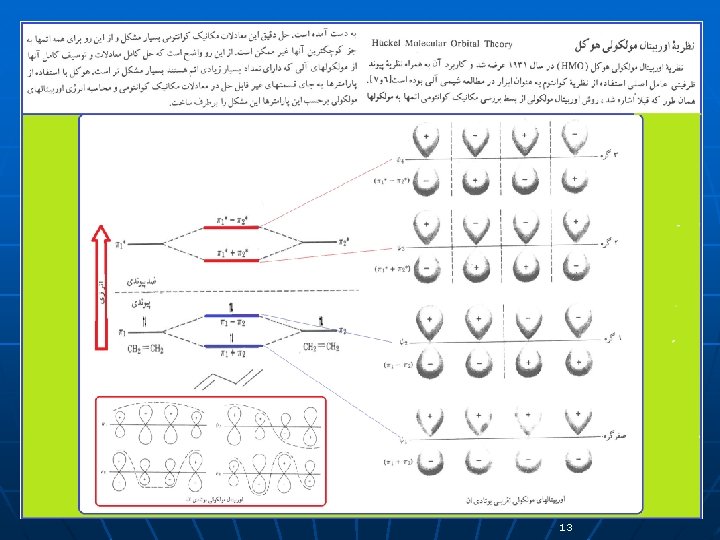
ﺗﺪﺍﺧﻞ ﺳﺎﺯﻧﺪﻩ Result Wave 1 Wave 2 ﺗﺪﺍﺧﻞ ﻣﺨﺮﺏ The characters c 1 and c 2 are the amounts of the contribution of atomic orbitals in the molecular orbitals.

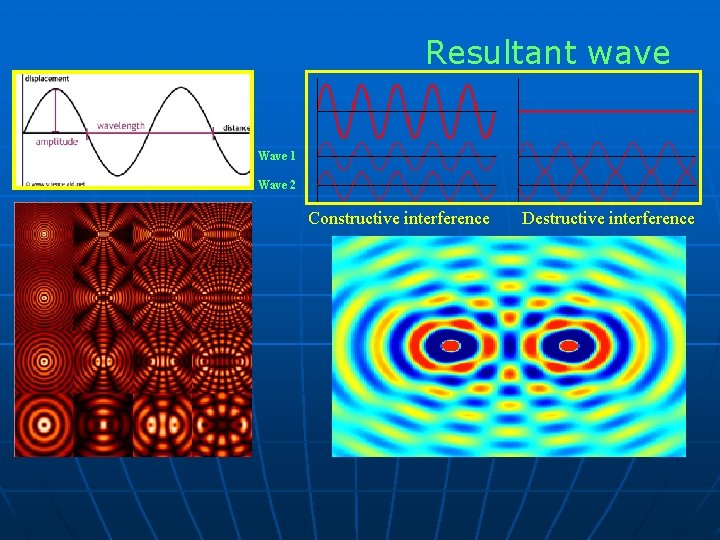
Resultant wave Wave 1 Wave 2 Constructive interference Destructive interference

10


12

13

Uncertainty Principle Heisenberg: In quantum mechanics, the uncertainty principle, also known as Heisenberg's uncertainty principle, is any of a variety of mathematical inequalities asserting a fundamental limit to the precision with which certain pairs of physical properties of a particle known as complementary variables, such as position x and momentum p, can be known simultaneously. Introduced first in 1927, by the German physicist "Werner Heisenberg", it states that the more precisely the position of some particle is determined, the less precisely its momentum can be known, and vice versa. The formal inequality relating the standard deviation of position Δx and the standard deviation of momentum Δm. v was derived by Earle Hesse Kennard later that year and by Hermann Weyl in 1928: Δx. Δm. v ≈ ћ/2 (ħ= 6. 62606957 E-34 m 2 kg/s, is the reduced Planck constant). 1) Heisenberg, W. (1927), "Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik", Zeitschrift für Physik (in German) 43 (3– 4): 172– 198. 2) Kennard, E. H. (1927), "Zur Quantenmechanik einfacher Bewegungstypen", Zeitschrift für Physik (in German) 44 (4– 5): 326. 3) Weyl, H. (1928), Gruppentheorie und Quantenmechanik, Leipzig: Hirzel, Germany. : ﺍﺻﻞ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻫﺎیﺰﻧﺒﺮگ ﺍیﻦ ﺍﺻﻞ ﻣﺪﻋی ﺍﺳﺖ. ﺑﻪ ﻋﻨﻮﺍﻥ ﺍﺻﻞ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻫﺎیﺰﻧﺒﺮگ ﺷﻨﺎﺧﺘﻪ ﺷﺪﻩ ﺍﺳﺖ ، ﺍﺻﻞ ﻋﺪﻡ ﻗﻄﻌیﺖ ، ﺩﺭ ﻣکﺎﻧیک کﻮﺍﻧﺘﻮﻣی کﻪ ﻫﺮ یک ﺍﺯ ﺍﻧﻮﺍﻉ ﻧﺎﺑﺮﺍﺑﺮﻫﺎی ﺭیﺎﺿی ﺑﺎ ﺟﻔﺖ ﺧﺎﺻی ﺍﺯ ﻭیژگی ﻓیﺰیکی ﺫﺭﺍﺕ ﺷﻨﺎﺧﺘﻪ ﺷﺪﻩ ﺑﻪ ﻋﻨﻮﺍﻥ ﻣﺘﻐیﺮﻫﺎی . کﻪ ﻣی ﺗﻮﺍﻥ ﺑﻪ ﻃﻮﺭ ﻫﻤﺰﻣﺎﻥ ﺷﻨﺎﺳﺎیی کﺮﺩ P ﻭ ﺍﻧﺪﺍﺯﻩ ﺣﺮکﺖ X ﻣﺎﻧﻨﺪ ﻣﻮﻗﻌیﺖ. ﺩﺭ ﺩﻗﺖ ﻣﺤﺪﻭﺩیﺖ ﺍﺳﺎﺳی ﺩﺍﺭﺩ ، ﻣکﻤﻞ ﺍیﻦ ﺍﺻﻞ ﺑیﺎﻥ ﻣی. ﺗﻮﺳﻂ ﻓیﺰیکﺪﺍﻥ آﻠﻤﺎﻧی "ﻭﺭﻧﺮ ﻫﺎیﺰﻧﺒﺮگ" ﻣﻌﺮﻓی ﺷﺪﻩ ﺍﺳﺖ ،1927 ﺍیﻦ ﺍﺻﻞ ﺍﻭﻟیﻦ ﺑﺎﺭ ﺩﺭ ﺳﺎﻝ . ﻣﻨﺠﺮ ﺑﻪ ﺩﻗﺖ کﻤﺘﺮﺩﺭ ﺍﻧﺪﺍﺯﻩ ﺣﺮکﺖ آﻦ ﺫﺭﻩ ﻣی ﺷﻮﺩ ﻭ ﺑﺎﻟﻌکﺲ ، ﺗﻌییﻦ ﺩﻗیﻘﺘﺮ ﻣﻮﻗﻌیﺖ ﻣکﺎﻧی ﺑﺮﺧی ﺍﺯ ﺫﺭﺍﺕ ، کﻨﺪ کﻪ E. H. ( ﺗﻮﺳﻂ Δm. v ) ( ﻭ ﺍﻧﺤﺮﺍﻑ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺍﺯ ﺍﻧﺪﺍﺯﻩ ﺣﺮکﺖ Δx) ﺍیﻦ ﻧﺎﺑﺮﺍﺑﺮی ﺩﺭ ﺍﻧﺤﺮﺍﻑ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺍﺯ ﻣﻮﻗﻌیﺖ ﻣکﺎﻧی : ﺑﻪ ﺩﺳﺖ آﻤﺪﻩ 1928 ﺩﺭ ﺳﺎﻝ H. Weyl ﻭ پﺲ ﺍﺯ ﺍﻭ ﺩﺭ ﻫﻤﺎﻥ ﺳﺎﻝ ﺗﻮﺳﻂ ،Kennard



17

18

19

20

21

22

End of Sessions 1 and 2 23
 Vitamin part1
Vitamin part1 Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Al razi facts
Al razi facts Razi iqbal
Razi iqbal Cycloalkanes
Cycloalkanes Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry Examples of isomers in chemistry
Examples of isomers in chemistry Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control David klein organic chemistry
David klein organic chemistry Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition What is the leveling effect organic chemistry
What is the leveling effect organic chemistry List of functional groups in order of priority
List of functional groups in order of priority Organic chemistry lab report sample
Organic chemistry lab report sample Organic chemistry conversion chart
Organic chemistry conversion chart Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Organic vs inorganic compounds
Organic vs inorganic compounds Kiliani fischer synthesis
Kiliani fischer synthesis Met et prop
Met et prop










































