What are the forces in a molecular structure
- Slides: 26

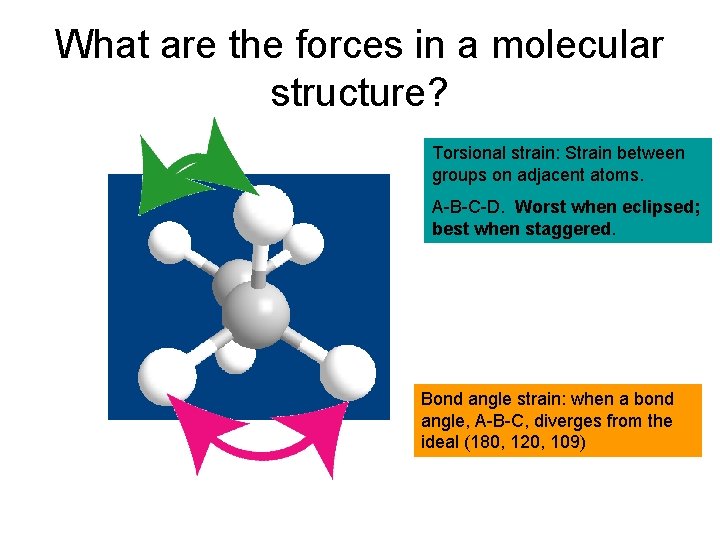
What are the forces in a molecular structure? Torsional strain: Strain between groups on adjacent atoms. A-B-C-D. Worst when eclipsed; best when staggered. Bond angle strain: when a bond angle, A-B-C, diverges from the ideal (180, 120, 109)

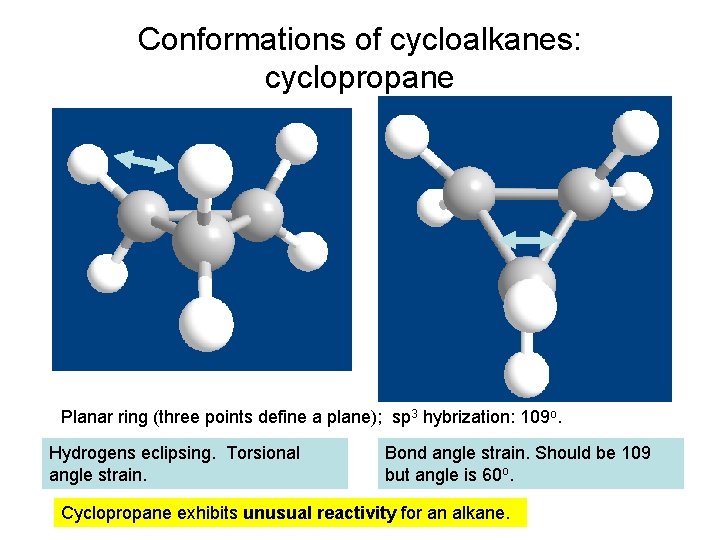
Conformations of cycloalkanes: cyclopropane Planar ring (three points define a plane); sp 3 hybrization: 109 o. Hydrogens eclipsing. Torsional angle strain. Bond angle strain. Should be 109 but angle is 60 o. Cyclopropane exhibits unusual reactivity for an alkane.

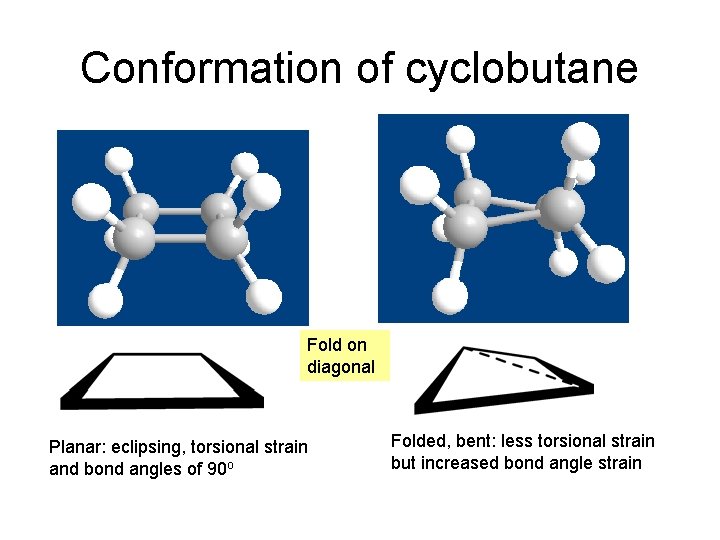
Conformation of cyclobutane Fold on diagonal Planar: eclipsing, torsional strain and bond angles of 90 o Folded, bent: less torsional strain but increased bond angle strain

Cyclobutane molecular dynamics

Cyclopentane

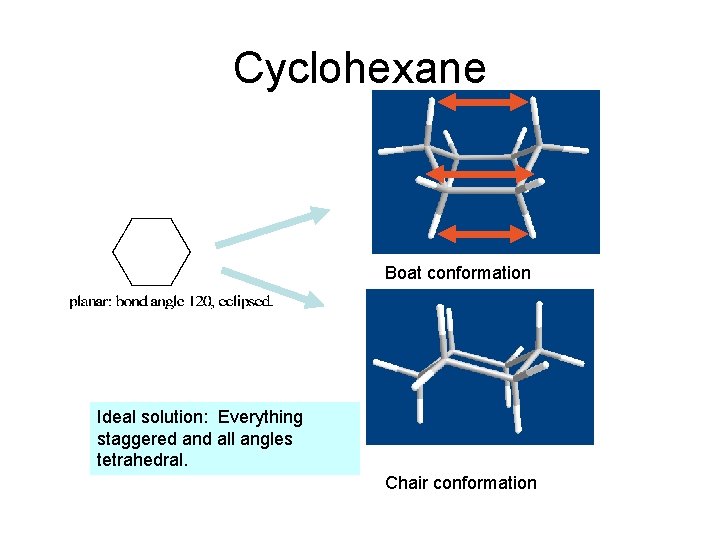
Cyclohexane Boat conformation Ideal solution: Everything staggered and all angles tetrahedral. Chair conformation

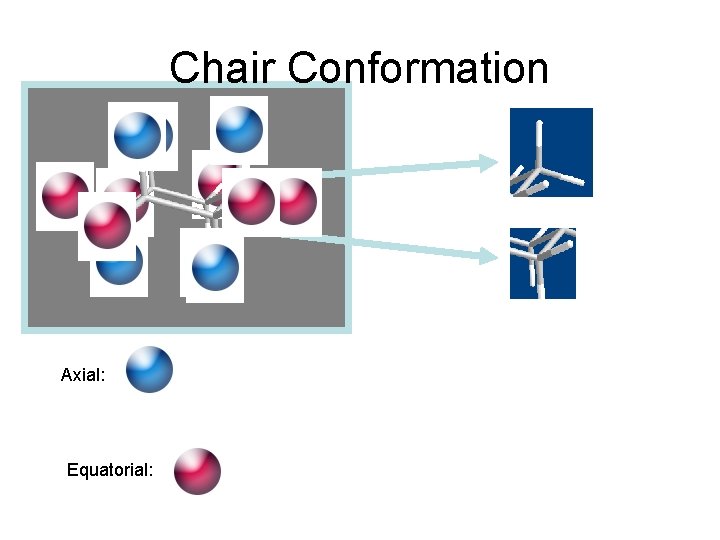
Chair Conformation Axial: Equatorial:

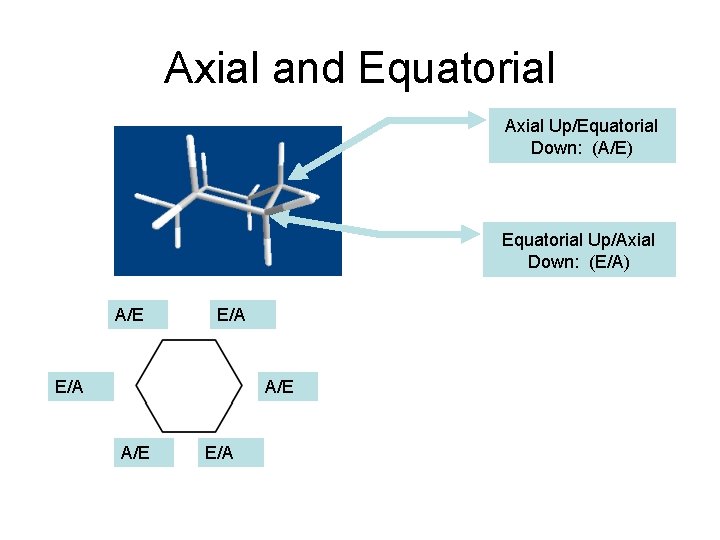
Axial and Equatorial Axial Up/Equatorial Down: (A/E) Equatorial Up/Axial Down: (E/A) A/E E/A

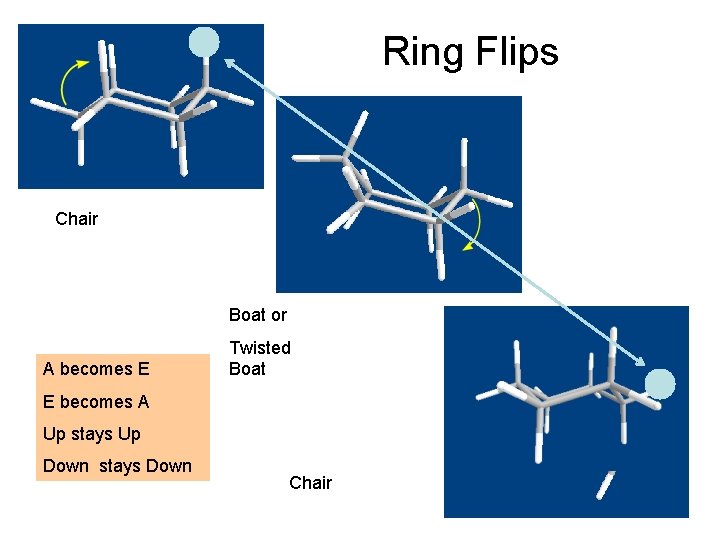
Ring Flips Chair Boat or A becomes E Twisted Boat E becomes A Up stays Up Down stays Down Chair

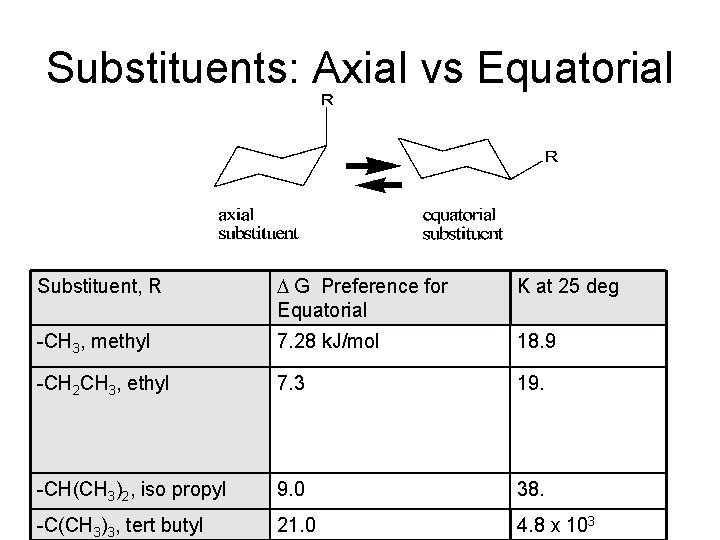
Substituents: Axial vs Equatorial Substituent, R D G Preference for Equatorial K at 25 deg -CH 3, methyl 7. 28 k. J/mol 18. 9 -CH 2 CH 3, ethyl 7. 3 19. -CH(CH 3)2, iso propyl 9. 0 38. -C(CH 3)3, tert butyl 21. 0 4. 8 x 103

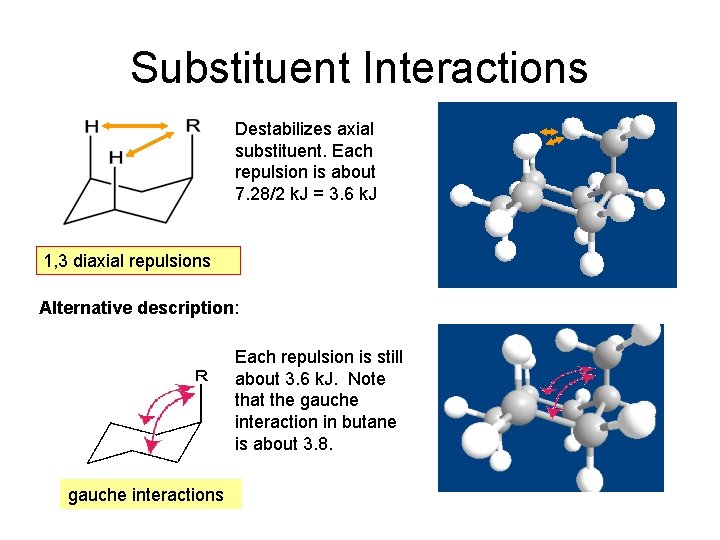
Substituent Interactions Destabilizes axial substituent. Each repulsion is about 7. 28/2 k. J = 3. 6 k. J 1, 3 diaxial repulsions Alternative description: Each repulsion is still about 3. 6 k. J. Note that the gauche interaction in butane is about 3. 8. gauche interactions

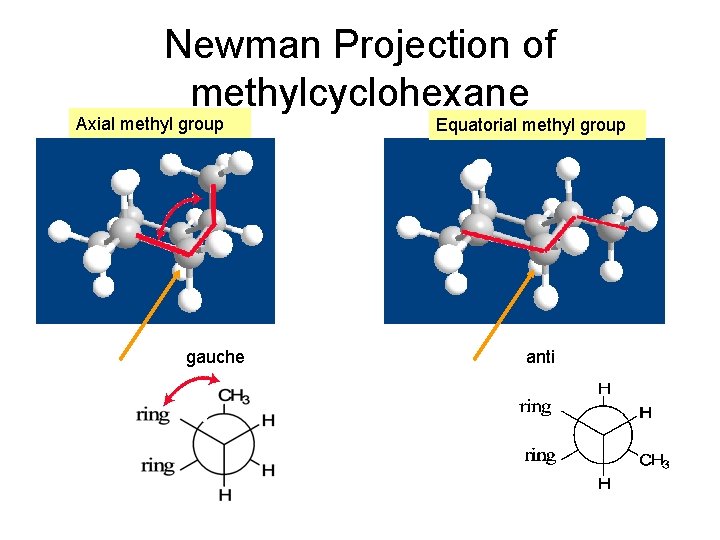
Newman Projection of methylcyclohexane Axial methyl group gauche Equatorial methyl group anti

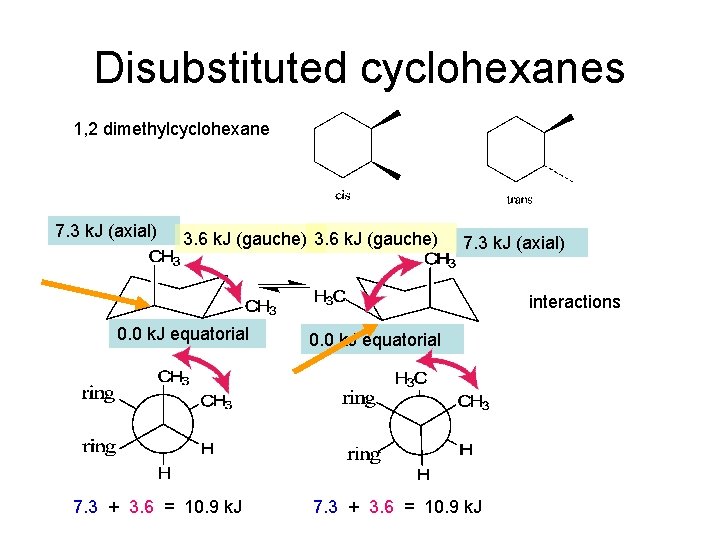
Disubstituted cyclohexanes 1, 2 dimethylcyclohexane 7. 3 k. J (axial) 3. 6 k. J (gauche) 7. 3 k. J (axial) interactions 0. 0 k. J equatorial 7. 3 + 3. 6 = 10. 9 k. J

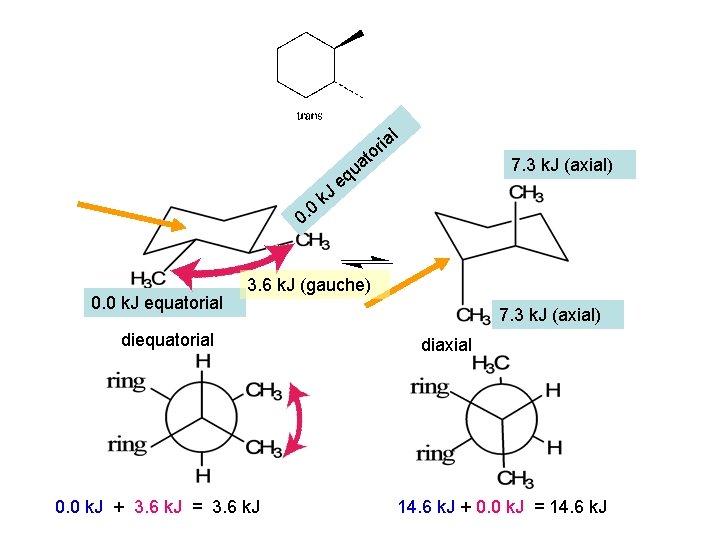
l ria to a u 0 0. 0 k. J equatorial k. J 7. 3 k. J (axial) eq 3. 6 k. J (gauche) diequatorial 0. 0 k. J + 3. 6 k. J = 3. 6 k. J 7. 3 k. J (axial) diaxial 14. 6 k. J + 0. 0 k. J = 14. 6 k. J

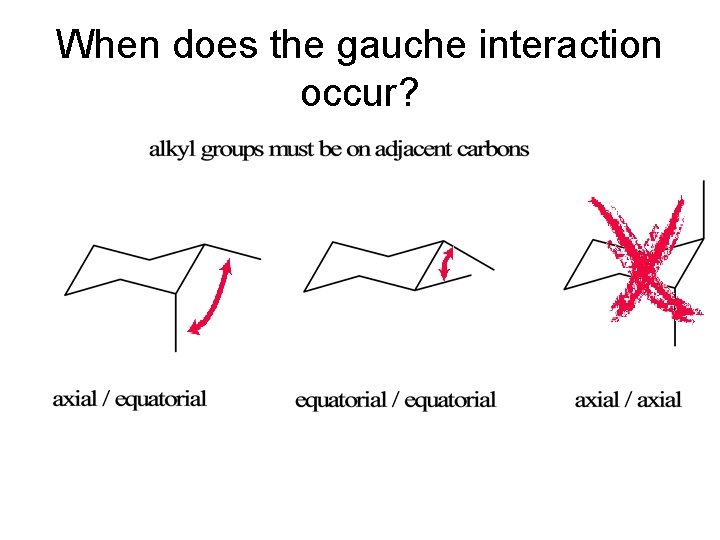
When does the gauche interaction occur?

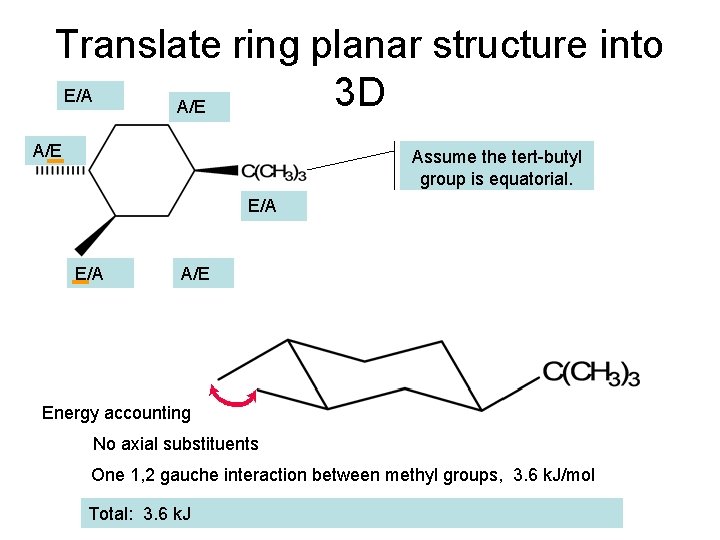
Translate ring planar structure into E/A 3 D A/E Assume the tert-butyl group is equatorial. E/A A/E Energy accounting No axial substituents One 1, 2 gauche interaction between methyl groups, 3. 6 k. J/mol Total: 3. 6 k. J

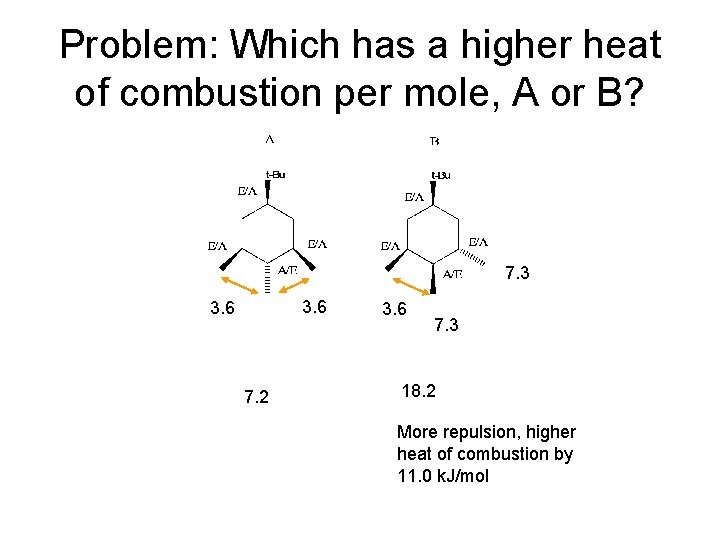
Problem: Which has a higher heat of combustion per mole, A or B? 7. 3 3. 6 7. 2 3. 6 7. 3 18. 2 More repulsion, higher heat of combustion by 11. 0 k. J/mol

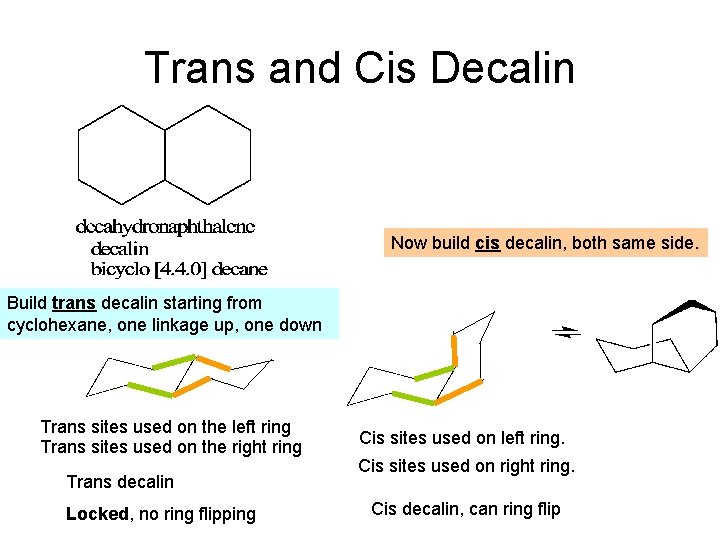
Trans and Cis Decalin Now build cis decalin, both same side. Build trans decalin starting from cyclohexane, one linkage up, one down Trans sites used on the left ring Trans sites used on the right ring Trans decalin Locked, no ring flipping Cis sites used on left ring. Cis sites used on right ring. Cis decalin, can ring flip

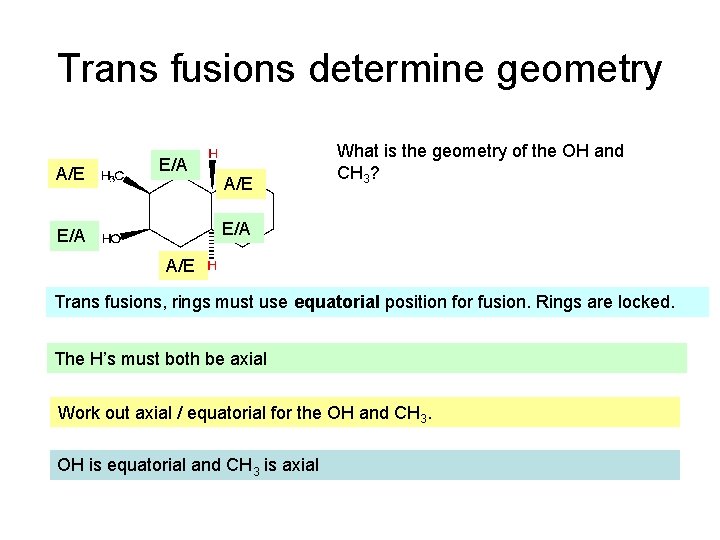
Trans fusions determine geometry A/E E/A A/E What is the geometry of the OH and CH 3? E/A A/E Trans fusions, rings must use equatorial position for fusion. Rings are locked. The H’s must both be axial Work out axial / equatorial for the OH and CH 3. OH is equatorial and CH 3 is axial

Inversion Point

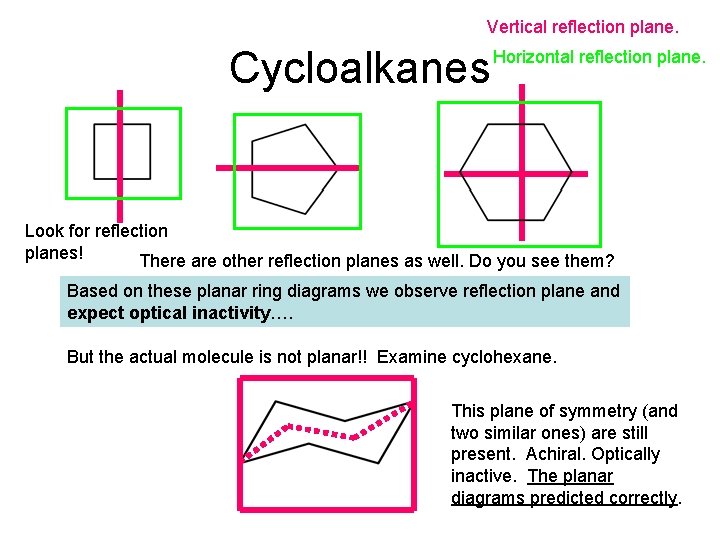
Vertical reflection plane. Cycloalkanes Horizontal reflection plane. Look for reflection planes! There are other reflection planes as well. Do you see them? Based on these planar ring diagrams we observe reflection plane and expect optical inactivity…. But the actual molecule is not planar!! Examine cyclohexane. This plane of symmetry (and two similar ones) are still present. Achiral. Optically inactive. The planar diagrams predicted correctly.

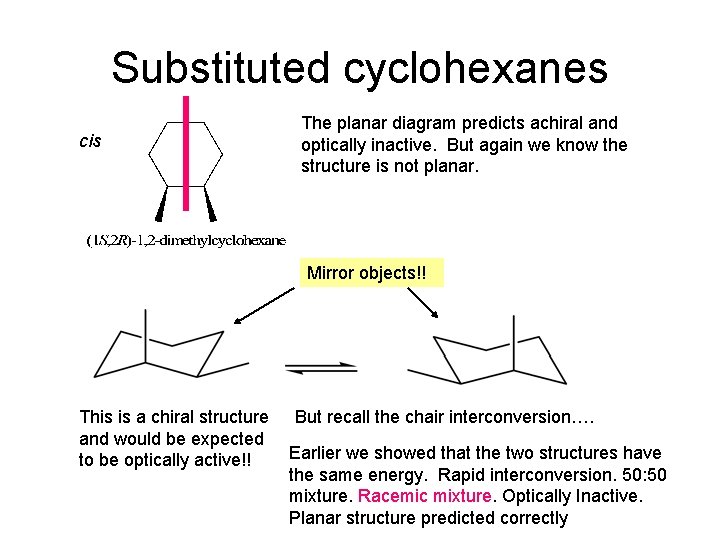
Substituted cyclohexanes cis The planar diagram predicts achiral and optically inactive. But again we know the structure is not planar. Mirror objects!! This is a chiral structure and would be expected to be optically active!! But recall the chair interconversion…. Earlier we showed that the two structures have the same energy. Rapid interconversion. 50: 50 mixture. Racemic mixture. Optically Inactive. Planar structure predicted correctly

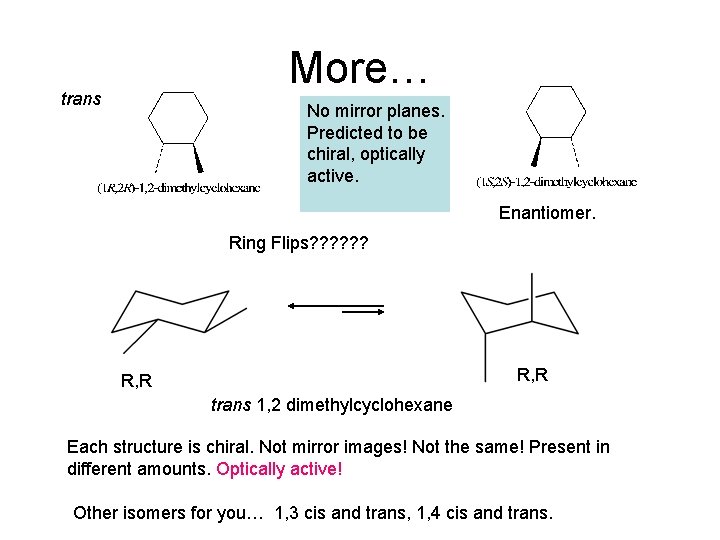
More… trans No mirror planes. Predicted to be chiral, optically active. Enantiomer. Ring Flips? ? ? R, R trans 1, 2 dimethylcyclohexane Each structure is chiral. Not mirror images! Not the same! Present in different amounts. Optically active! Other isomers for you… 1, 3 cis and trans, 1, 4 cis and trans.

Resolution of mixture into separate enantiomers. Mixtures of enantiomers are difficult to separate because the enantiomers have the same boiling point, etc. The technique is to convert the pair of enantiomers into a pair of diastereomers and to utilize the different physical characteristics of diastereomers. Formation of diastereomeric salts. Racemic mixture of anions allowed to form salts with pure cation enantiomer. Racemic mixture reacted with chiral enzyme. One enantiomer is selectively reacted. Racemic mixture is put through column packed with chiral material. One enantiomer passes through more quickly.

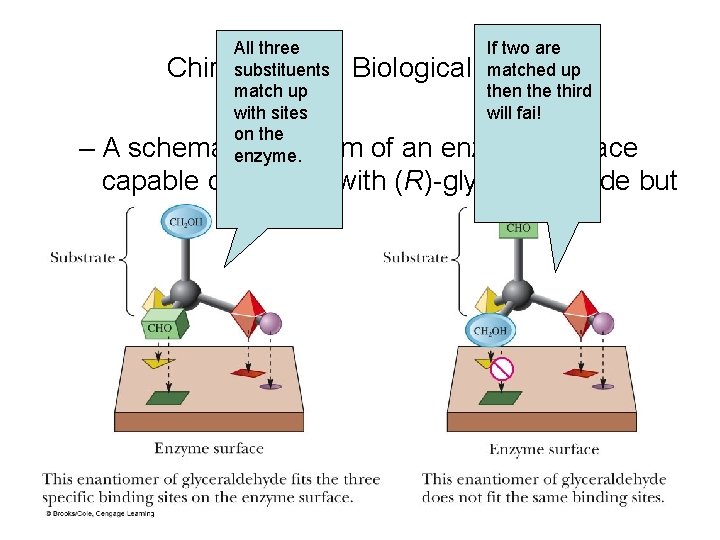
All three If two are substituents matched up Chirality in the Biological World match up then the third with sites will fai! on the schematic diagram of an enzyme surface enzyme. –A capable of binding with (R)-glyceraldehyde but not with (S)-glyceraldehyde.

Sumber • academic. brooklyn. cuny. edu/. . . /chem%20 51%20 Lecture%20 Slides/Lecture%2004. ppt • 31 Januari 2010 jam 22. 10 • (Alkane filetype: ppt)