VSEPR Theory Molecular Structure Molecular structure the threedimensional



















- Slides: 48

VSEPR Theory

Molecular Structure • Molecular structure – the threedimensional arrangement of atoms in a molecule

VSEPR Theory • VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) • A model for describing the shapes of molecules whose main postulate is that the structure around a given atom is determined by minimizing the electron pair repulsion • Therefore, the electrons and elements bonded to the central atom want to be as far apart as possible

VSEPR Steps 1. Draw the Lewis structure for the molecule 2. Count the total number of things that are around the central atom to determine the electron pair geometry 3. Imagine that the lone pairs of electrons are invisible and describe the molecular shape

SORRY… • Yes…you must memorize the main shapes and bond angles


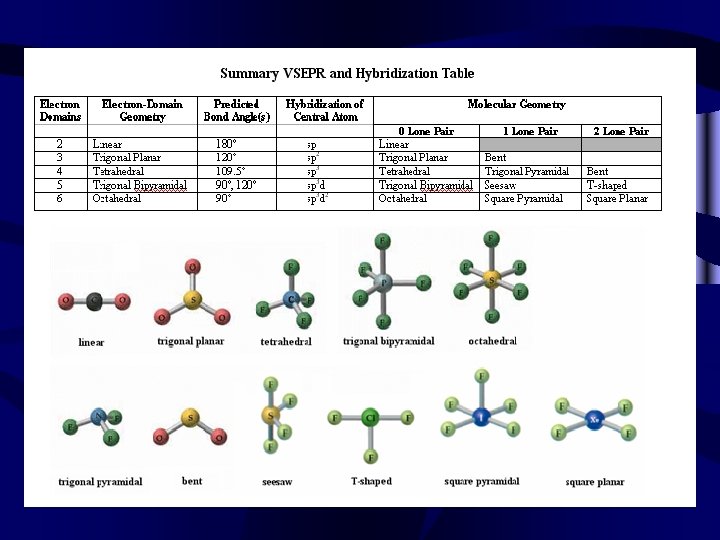
2 Electron Pairs • If there are 2 things attached to the central atom, the shape is linear • 220

3 Electron Pairs • If there are 3 electron pairs or bonds the shape will be trigonal planar • 330 • Bond angle = 120°

3 electron pairs • Now imagine that you have 3 electron pairs, but one is just a lone pair (invisible) what would it look like then? • 321

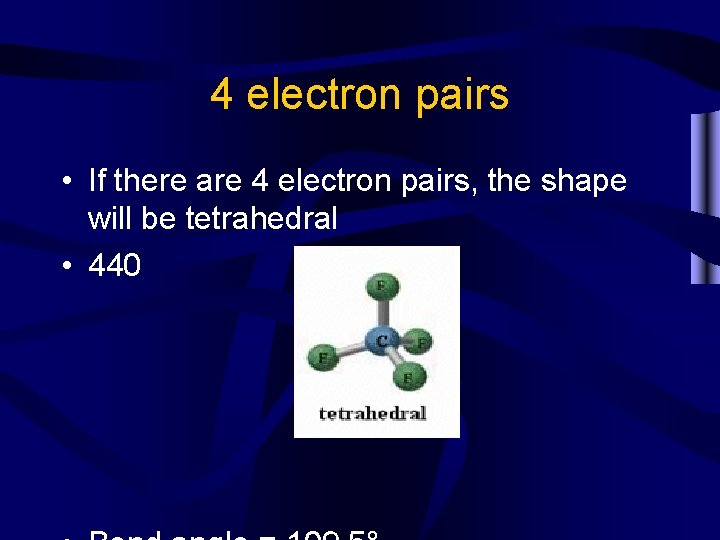
4 electron pairs • If there are 4 electron pairs, the shape will be tetrahedral • 440

4 electron pairs • What if 1 of the electron pairs is a lone pair (invisible)? What would it look like then? • 431

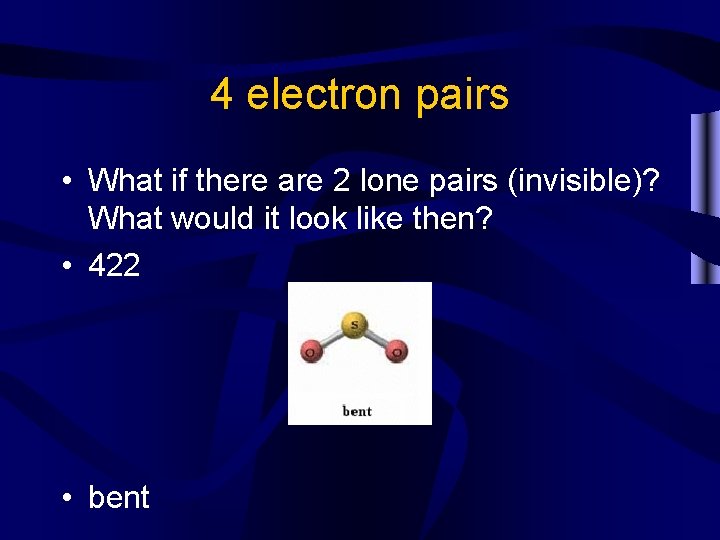
4 electron pairs • What if there are 2 lone pairs (invisible)? What would it look like then? • 422 • bent

5 electron pairs • If there are 5 electron pairs the shape will be Trigonal Bipyramidal • 550 • Bond angles = 90º & 120º

5 electron pairs • What if there is 1 lone pair (invisible) • 540 • Seesaw

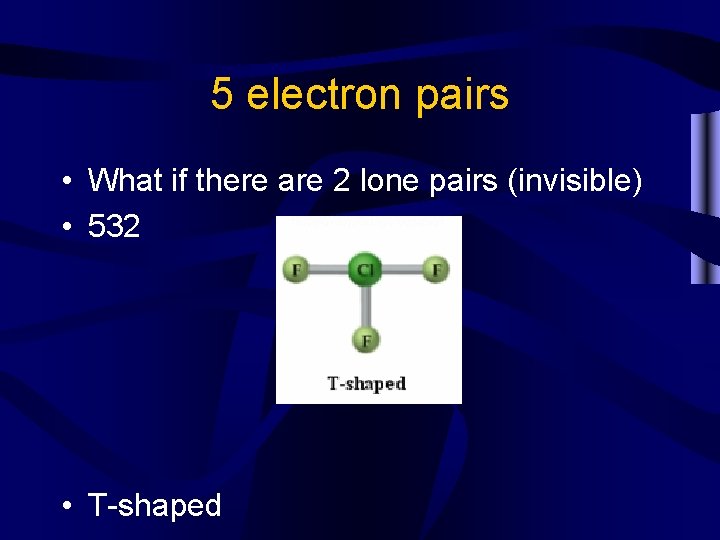
5 electron pairs • What if there are 2 lone pairs (invisible) • 532 • T-shaped

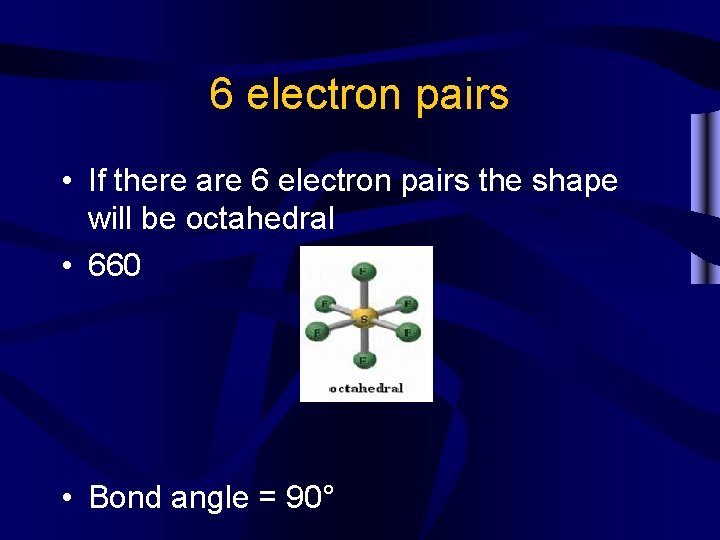
6 electron pairs • If there are 6 electron pairs the shape will be octahedral • 660 • Bond angle = 90°

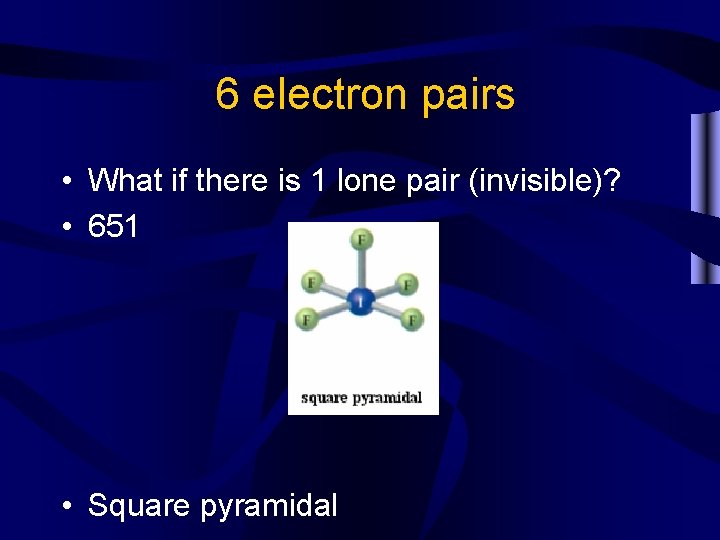
6 electron pairs • What if there is 1 lone pair (invisible)? • 651 • Square pyramidal

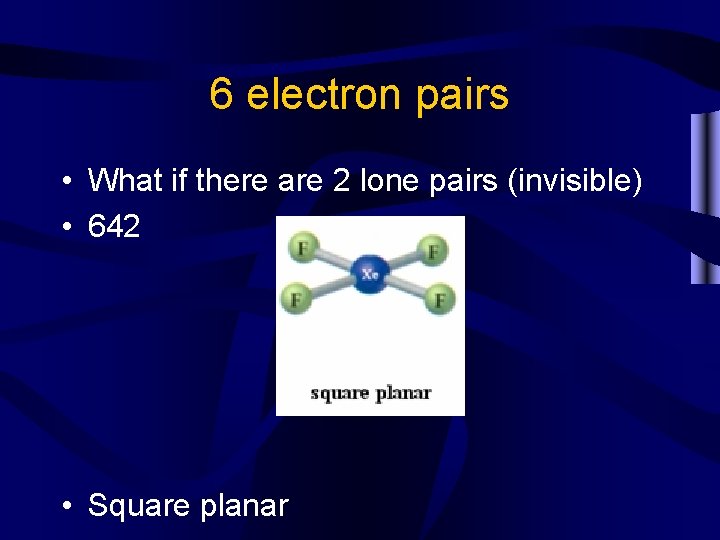
6 electron pairs • What if there are 2 lone pairs (invisible) • 642 • Square planar


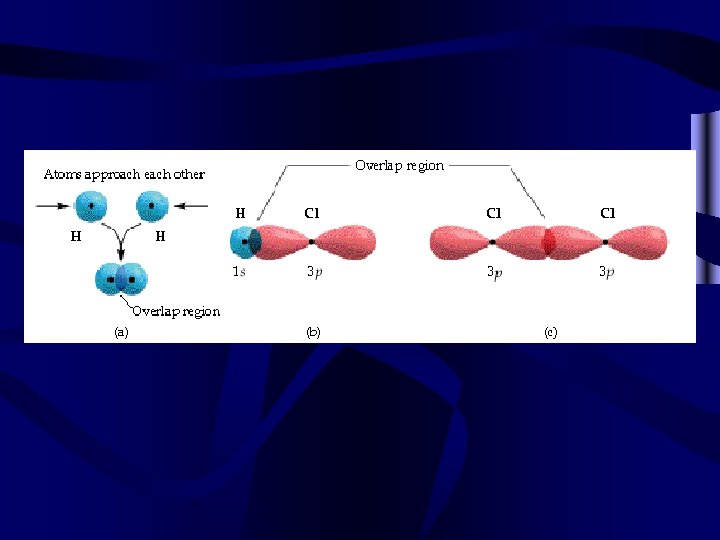
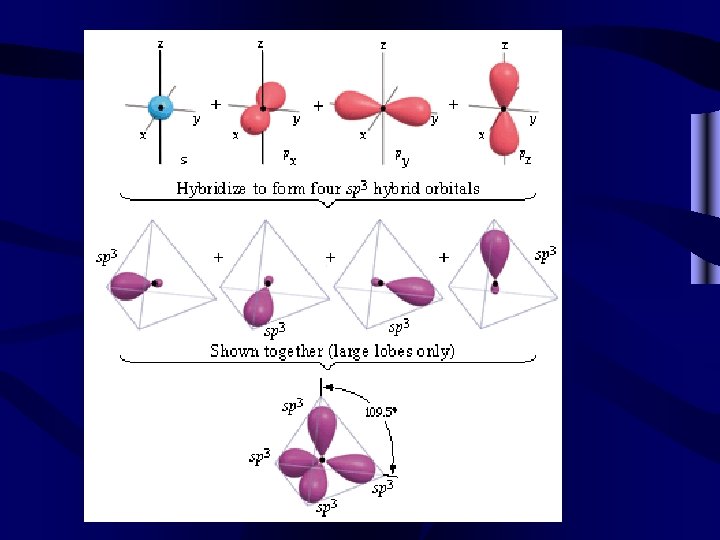
Hybridization • Look at the orbital notation for the carbon atom on the right. • There appear to be only two electrons available for bonding with other atoms. • However, one carbon will bond equally with four hydrogens as in methane, CH 4. 2 s 2 p X X C X X

Hybridization of the carbon atom • The orbitals combine an s orbital and three p orbitals to form four equal sp 3 hybrid orbitals. • Each hybrid orbital can bond with a hydrogen atom. sp 3 hybrid orbitals H X 0 X X H 0 C 0 H X 0 H





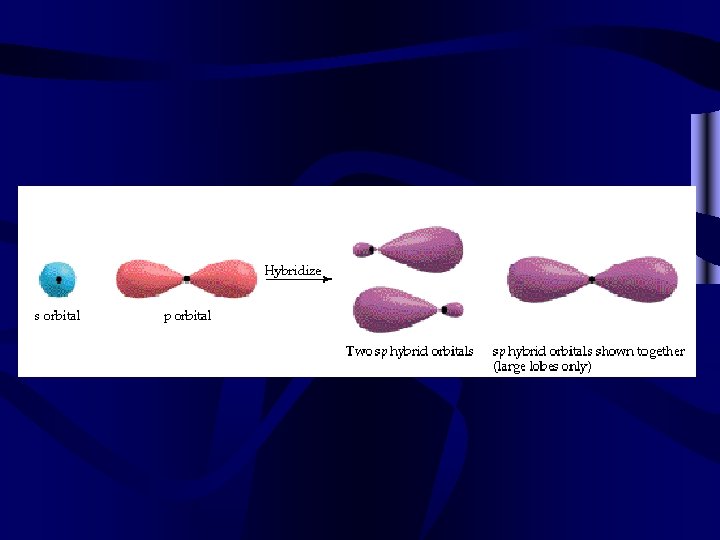
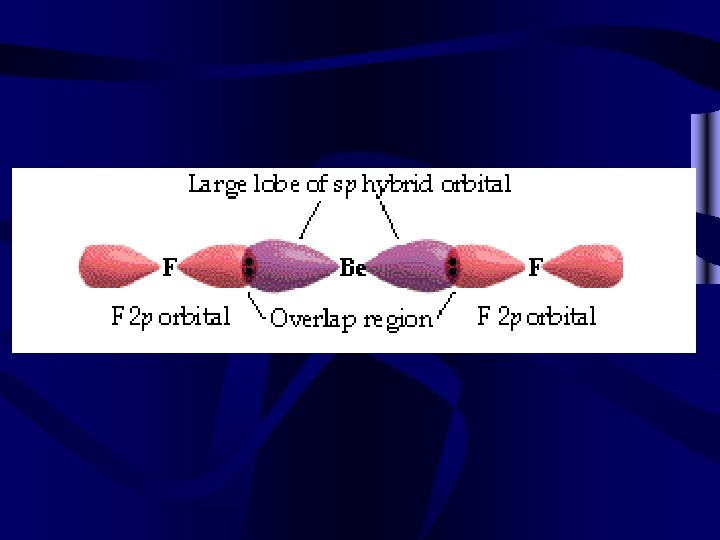
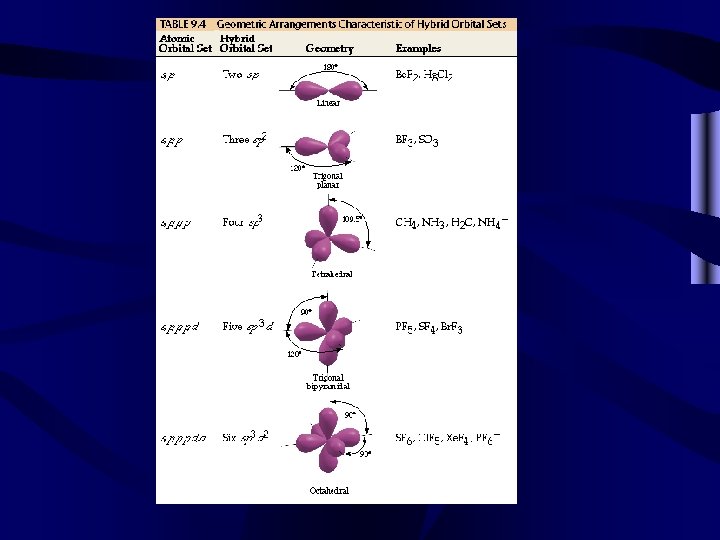
Most molecular bonding involves hybrid orbitals. • Two equal bonds are formed by a s and a p orbital-2 sp hybrids • Three equal bonds are formed by a s and 2 p orbitals-3 sp 2 hybrids • Four equal bonds are formed by a s and 3 p orbitals-4 sp 3 hybrids • Five equal bonds are formed by a s, 3 p and a d orbital-5 sp 3 d hybrids • Six equal bonds are formed by a s, 3 p and 2 d orbitals-6 sp 3 d 2 hybrids

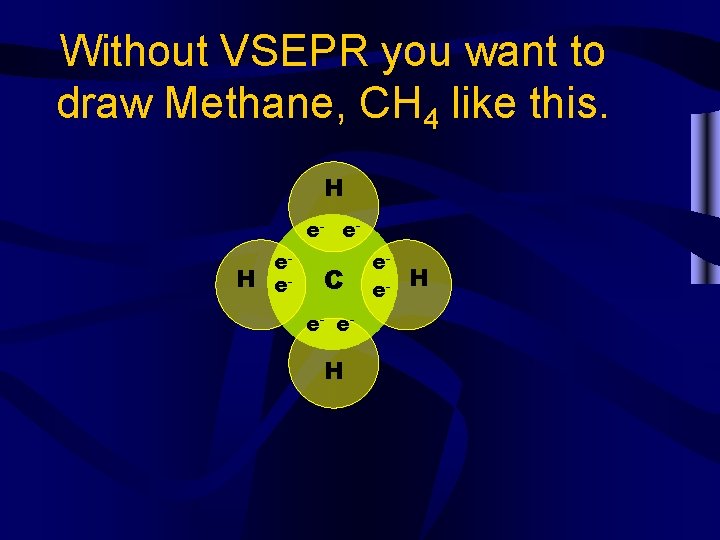
Without VSEPR you want to draw Methane, CH 4 like this. H e- e- H ee- C e- e- H ee- H

However, this isn’t VSEPR. • This looks good in 2 dimensions, all angles are 90° but the molecule is 3 D. • The electron pairs aren’t as far apart as possible. • From the side we see that some are 180 °. This doesn’t fit VSEPR. H e- e- H ee C e- e H e- e- H H

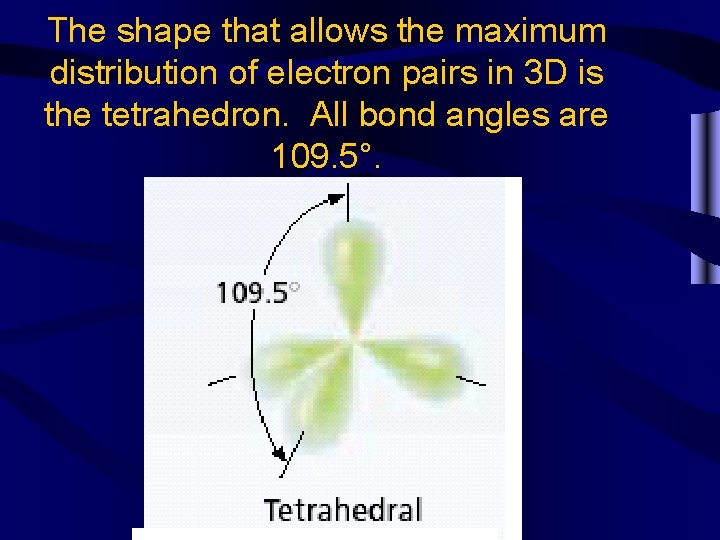
The shape that allows the maximum distribution of electron pairs in 3 D is the tetrahedron. All bond angles are 109. 5°.

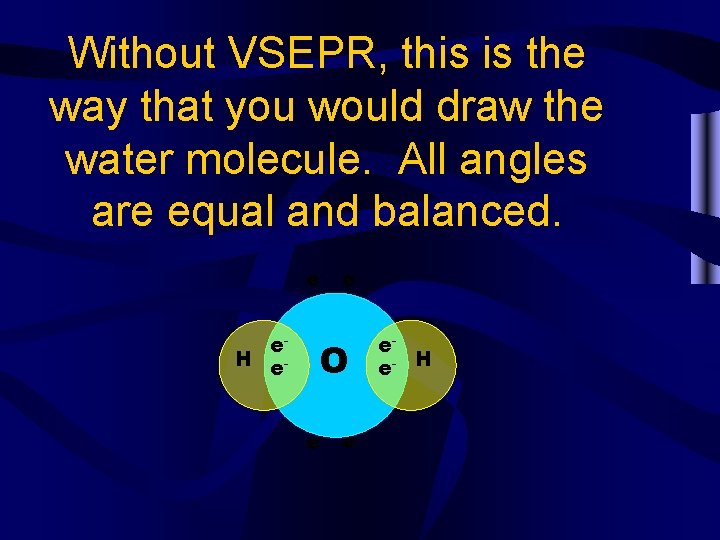
Without VSEPR, this is the way that you would draw the water molecule. All angles are equal and balanced. e- ee. H e- O e- e- ee- H

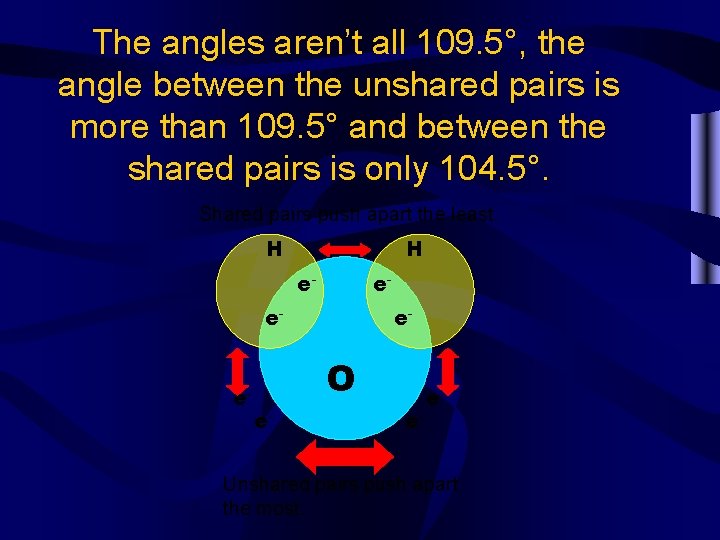
Due to the unequal repulsion of the electrons the shape looks more like this. Why? H H e- e- e- O e- e-

The angles aren’t all 109. 5°, the angle between the unshared pairs is more than 109. 5° and between the shared pairs is only 104. 5°. Shared pairs push apart the least. H H e- e- e- O e- e- e- Unshared pairs push apart the most.

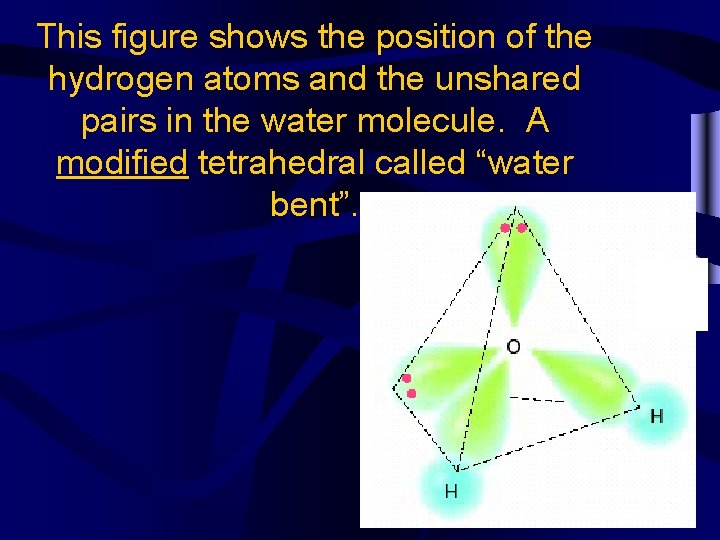
This figure shows the position of the hydrogen atoms and the unshared pairs in the water molecule. A modified tetrahedral called “water bent”.

VSEPR Basic Shapes • Linear shapes occur with 1 or 2 shared pairs and sp hybrids. • Trigonal planar shapes occur with sp 2 hybrids. • Tetrahedral shapes are based on sp 3 hybrids. • Trigonal bipyrimidals are based on sp 3 d hybrids. • Octahedrals are based on sp 3 d 2 hybrids. • All shapes are based upon these 5 shapes.

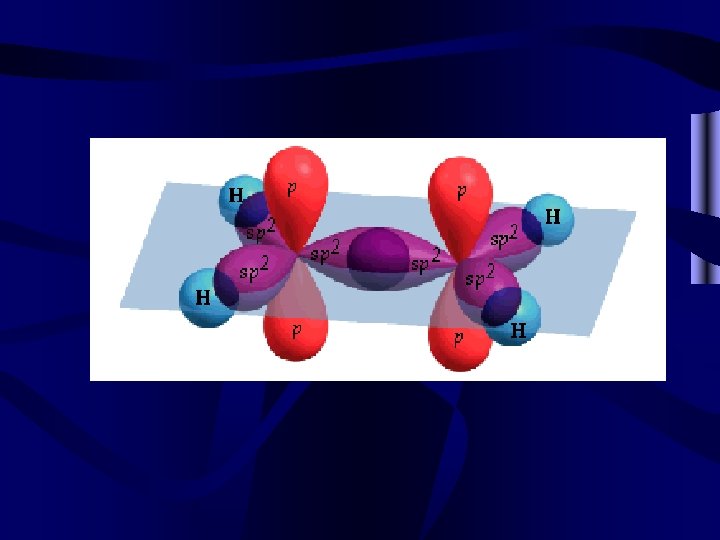
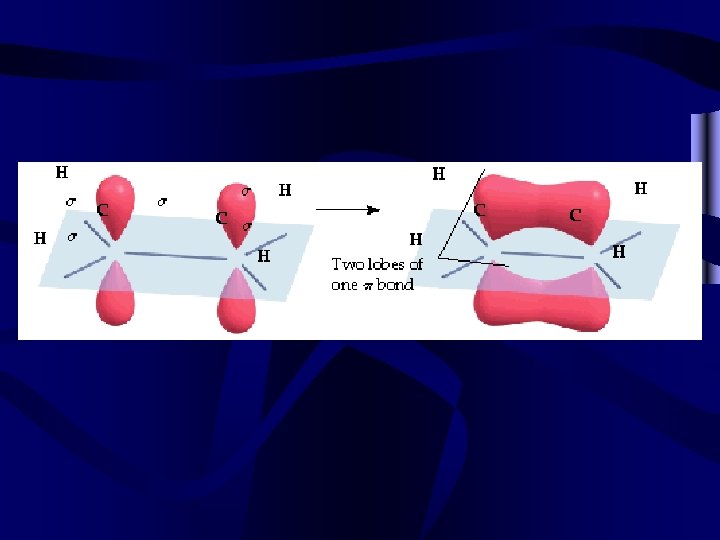
Organic molecules • Often organic molecules (generally those compounds containing carbon, hydrogen, and sometimes oxygen) form multiple bonds. These multiple bonds involve more than one pair of e-. For V. S. E. P. R. , count all multiple bonds as a single pair of e-. • When an organic compound has multiple bonds it is called unsaturated. This means that it doesn’t have the max. number of H for the number of carbons.

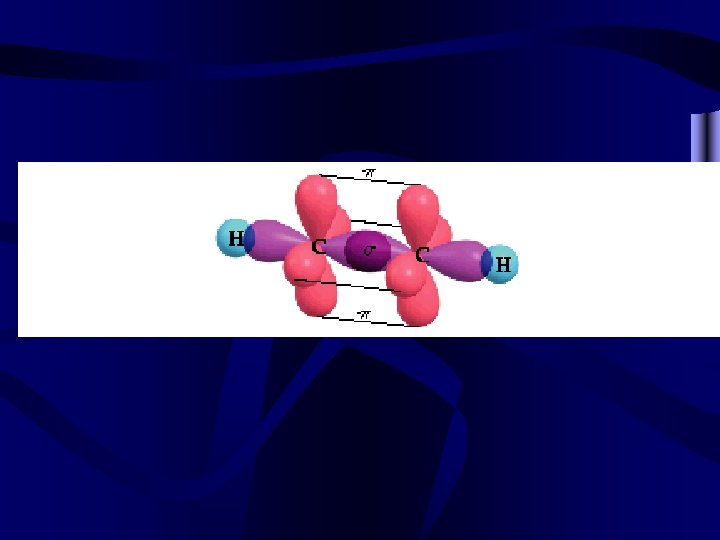
and bonds • All bonds contain a bond that occurs directly between the nuclei involved. This bond is a bond. • Multiple bonds also overlap above and out to the side of bonds. Bonds that occur outside of a line drawn between the nuclei are called bonds. • Double bonds have 1 and 1 . Triple bonds have 1 and 2 bonds.




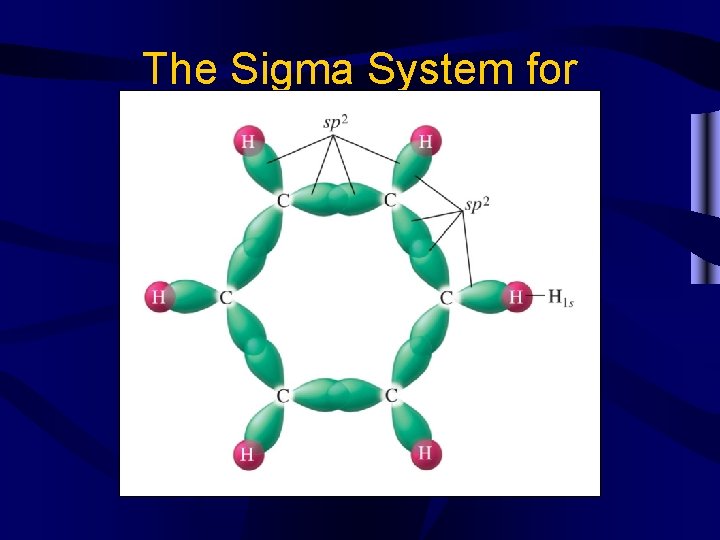
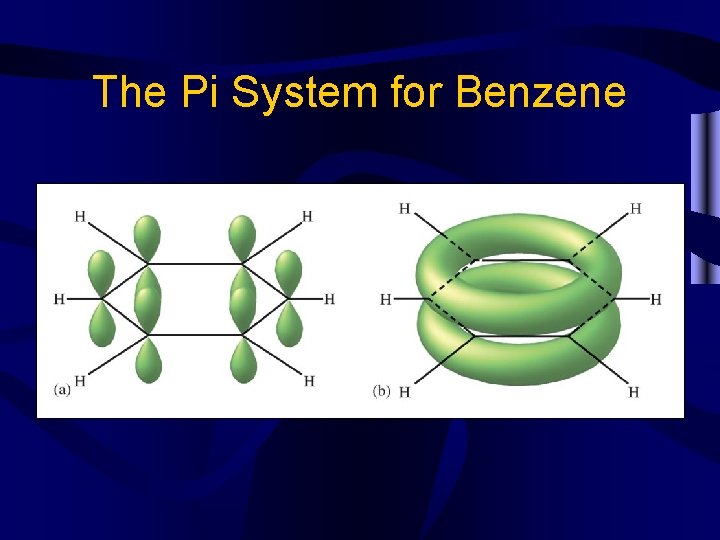
Other bonds • Conjugated systems occur when multiple p orbitals overlap, as in benzene C 6 H 6. They are very stable. • Coordinate covalent bonds are bonds where both e- in the shared pair come from one atom.

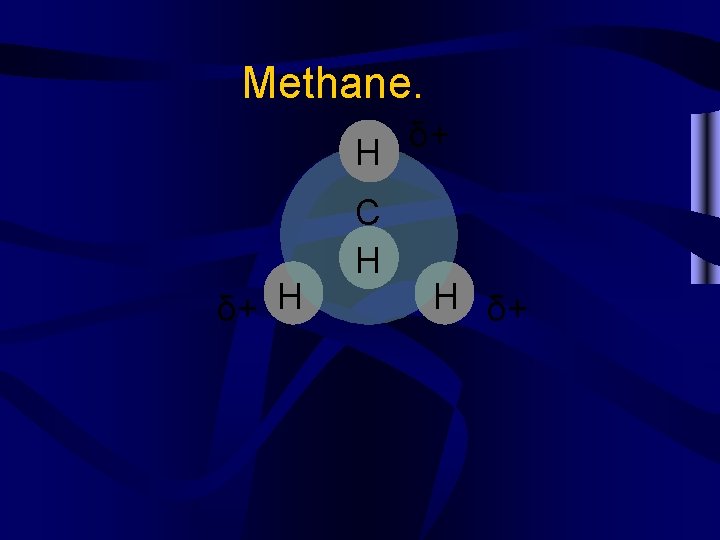
Polar bond vs. Polar molecule • Every year students have trouble with the difference between these two terms. • Polar bonds refer to the difference in electronegativities between two atoms. Between 0. 3 and about 1. 7 -polar bond. • Polar molecules refer to the shape of a molecule. If the partial charges aren’t evenly distributed, it’s a polar molecule.

Methane. H δ+ H C H δ+

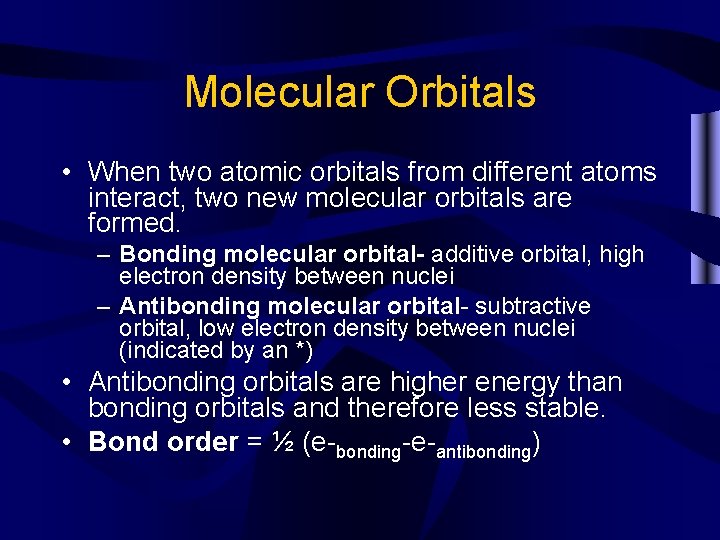
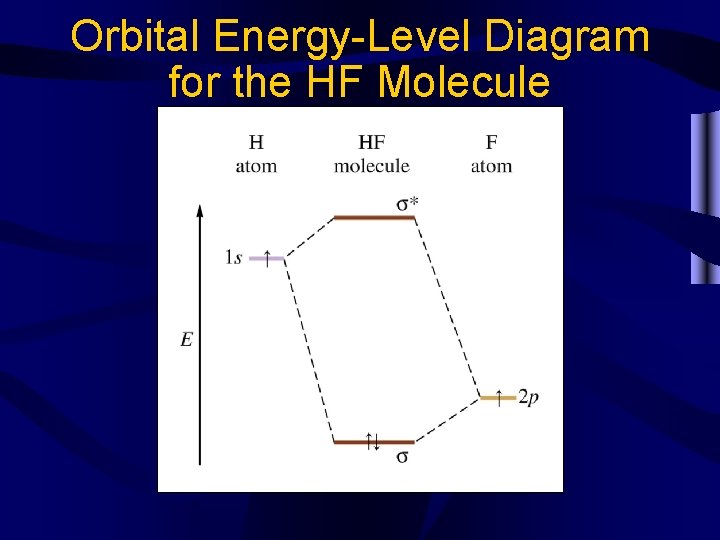
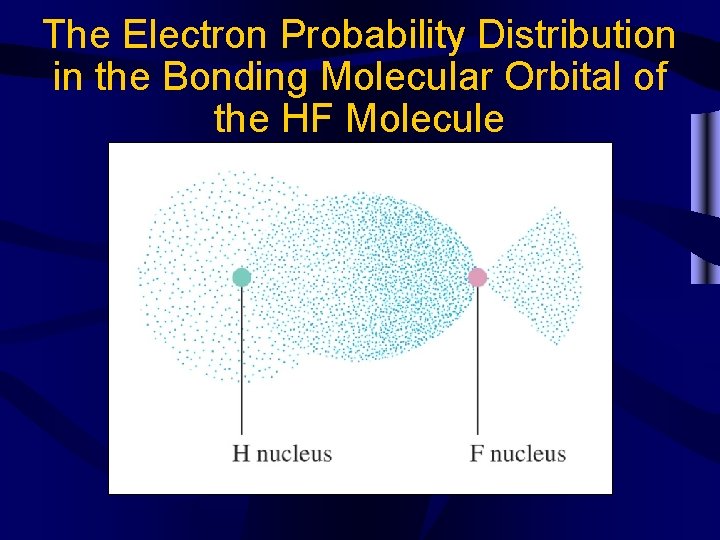
Molecular Orbitals • When two atomic orbitals from different atoms interact, two new molecular orbitals are formed. – Bonding molecular orbital- additive orbital, high electron density between nuclei – Antibonding molecular orbital- subtractive orbital, low electron density between nuclei (indicated by an *) • Antibonding orbitals are higher energy than bonding orbitals and therefore less stable. • Bond order = ½ (e-bonding-e-antibonding)

Orbital Energy-Level Diagram for the HF Molecule

The Electron Probability Distribution in the Bonding Molecular Orbital of the HF Molecule

The Sigma System for Benzene

The Pi System for Benzene
