Treatment of hyperthyroidism Fereidoun Azizi M D Research


































- Slides: 46


Treatment of hyperthyroidism Fereidoun Azizi, M. D. Research Institute for Endocrine Sciences Shaheed Beheshti University of Medical Sciences Tehran, I. R. Iran The First Arab-Iran Endocrine Congress April 9 -10, 2008, Damascus, Syria


Introduction Treatment of basic pathogenic factors in thyrotoxicosis is only possible in rare conditions, such as thyrotropin secreting pituitary tumors, iatrogenic thyrotoxicosis and struma ovarii; existing therapies are only palliative. Treatment of thyrotoxicosis is designed to restrain thyroid hormone synthesis (antithyroid drugs) or to reduce the quantity of thyroid tissue (radioiodine and surgery).

Drugs with antithyroid action 1. Thiocyanate and percholorate inhibit thyroid iodine transport. 2. Iodine and iodine-containing agents and lithium inhibit hormone release 3. The iodine-containing contrast agent sodium ipodate also inhibits peripheral T 3 production from thyroxine T 4 4. Dexamethasone inhibits the glandular secretion of thyroid hormones and the peripheral conversion of T 4 to T 3 However, their use in the treatment of thyrotoxicosis is limited.



Pharmacology of Thionamides Rapidly absorbed from gastro-intestinal tract showing a peak in concentration in blood within one to two hours after ingestion. Methimazole is essentially free in serum, whereas 80 to 90 percent of propylthiouracil is bound to albumin. The thyroidal concentration of thionamide, is many fold of that in the serum The half-life of methimazole in plasma is 6 -8 hours and that of propylthiouracil is about 1 -2 hours. A single dose of methimazole may exert an antithyroid effect for longer than 24 hours.

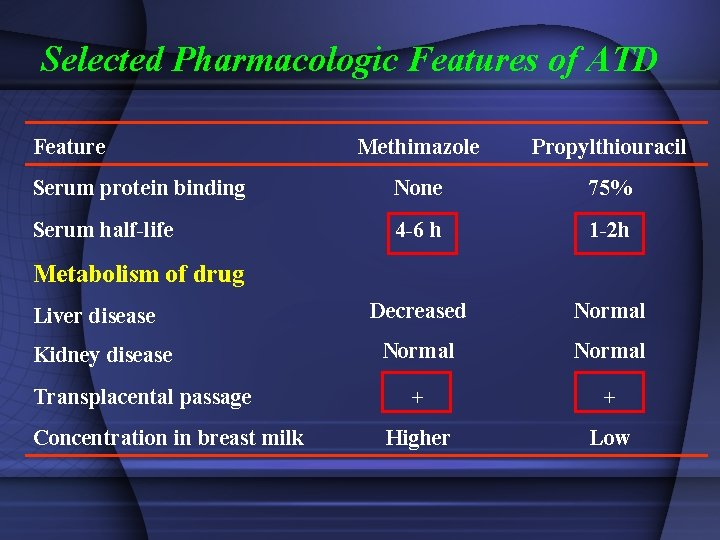
Selected Pharmacologic Features of ATD Feature Methimazole Propylthiouracil Serum protein binding None 75% Serum half-life 4 -6 h 1 -2 h Decreased Normal + + Higher Low Metabolism of drug Liver disease Kidney disease Transplacental passage Concentration in breast milk

Causes of Thyrotoxicosis • Diffuse toxic goiter • Toxic multinodular goiter • Toxic adenoma • Iatrogenic • Iodine excess • Postpartum thyroiditis • Strauma overaii • TSH- secreting Subacute thyroiditis Spontaneously resolving Trophoblastic tumors Hashitoxicosis Metastatic thyroid ca

Duration of therapy with thionamides 1. Preparation for ablation of the thyroid: the duration of therapy is up to the time that patient attains euthyroidism. Methimazole is the preferred agent for attaining euthyroidism before radioiodine therapy. 2. Treatment of diffuse toxic goiter.

Use of Thionamides Compared to propylthiouracil, methimazole induces more speedy improvement in serum levels of T 4 and T 3. This effect, added to once or twice daily doses and better patient adherence, make this drug more desirable than propylthiouracil.



Summary of data of thionamide therapy 1. Significant improvement in the rate of relapse in patients treated for 18 months, as compared with those treated for 6 months 2. Up to four years of follow up did not find that treatment for longer than one year had any effect on relapse rate 3. Longer courses of therapy in children are accompanied by more likelihood of remission 4. There is no theoretical reason why patients whose disease is well controlled with a maintenance dose of thionamide drug could not continue with such a therapy indefinitely 5. A recent study of continuous methimazole therapy, showed that adapting such a treatment strategy may be beneficial for many patients.

Factors favoring relapse of thyrotoxicosis after treatment of Graves’ disease with thionamides More consistent factors: § § § Severe degree of thyrotoxicosis Large goiter Lack of decrease of goiter size during therapy High T 3 to T 4 ratio in serum Higher baseline levels of anti-TSH receptor antibodies Lack of normalization of serum TSH Inconsistent factors: § Sex, age, cigarette smoking, duration of symptoms before diagnosis, presence of ophthalmopathy, psychiatric disorders, (depression, hypochondriasis, paranoia, mental fatigue) and problems of daily life Azizi F. Exper Opin Drug Saf 2006; 5: 107 -116

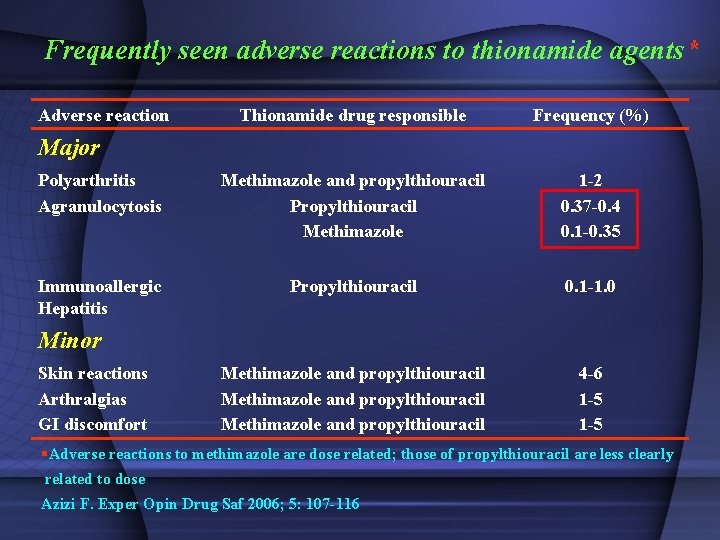
Frequently seen adverse reactions to thionamide agents* Adverse reaction Thionamide drug responsible Frequency (%) Polyarthritis Agranulocytosis Methimazole and propylthiouracil Propylthiouracil Methimazole 1 -2 0. 37 -0. 4 0. 1 -0. 35 Immunoallergic Hepatitis Propylthiouracil 0. 1 -1. 0 Methimazole and propylthiouracil 4 -6 1 -5 Major Minor Skin reactions Arthralgias GI discomfort §Adverse reactions to methimazole are dose related; those of propylthiouracil are less clearly related to dose Azizi F. Exper Opin Drug Saf 2006; 5: 107 -116

Comparison of various properties of common thianomid drugs Property Methimazole propylthiouracil Longer half life Once or twice daily administration More speedy improvement is serum T 4 and T 3 Better patient compliance Lower treatment failure with radioiodine treatment Major side effects: Agranulocytosis + + Polyartritis + + Immunoallergic hepatitis - + Safety in pregnancy + + Safety in lactation + + Azizi F. Exper Opin Drug Saf 2006; 5: 107 -116

Special considerations Thionamide agents have been the treatment of choice for thyrotoxicosis during the pregnancy and postpartum periods. However use of these drugs during these important periods of woman’s life needs special considerations.


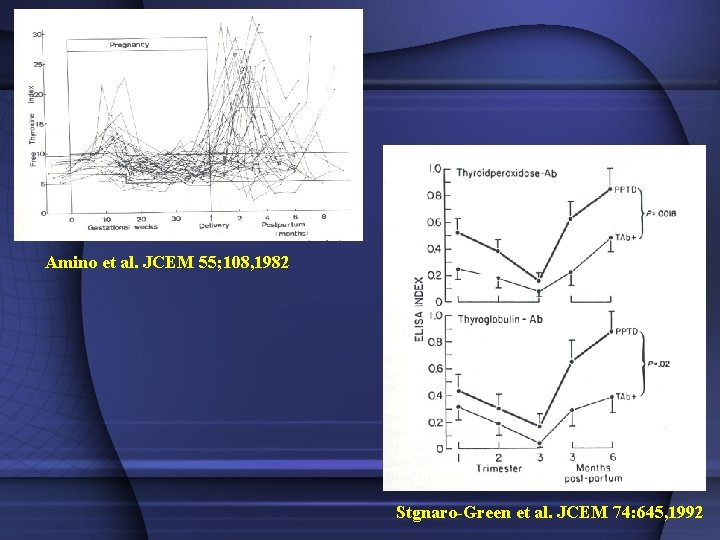
Thyrotoxicosis in postpartum period • Profound autoimmune modification after delivery Graves’ disease – Onset: 12 -46% of all childbearing age – Recurrence – Exacerbation • • Thyrotoxic phase of postpartum thyroiditis Graves’ disease + postpartum thyroiditis Subacute thyroiditis Toxic nodular goiter

Amino et al. JCEM 55; 108, 1982 Stgnaro-Green et al. JCEM 74: 645, 1992

Management of Postpartum thyrotoxicosis • Postpartum, subacute, sporadic silent No treatment for thyrotoxicosis • Graves’ disease – Antithyroids – Radiiodine – Thyroidectomy

Thionamides and Lactation For many years breast-feeding was forbidden if antithyroid drugs were being used, because both methimazole (MMI) and propylthiouracil are transferred into the breast milk. It was shown that only small amounts of propylthiouracil are found in milk, therefore, lactating women with Graves’ disease receiving propylthiouracil have been advised that breasdfeeding is safe.

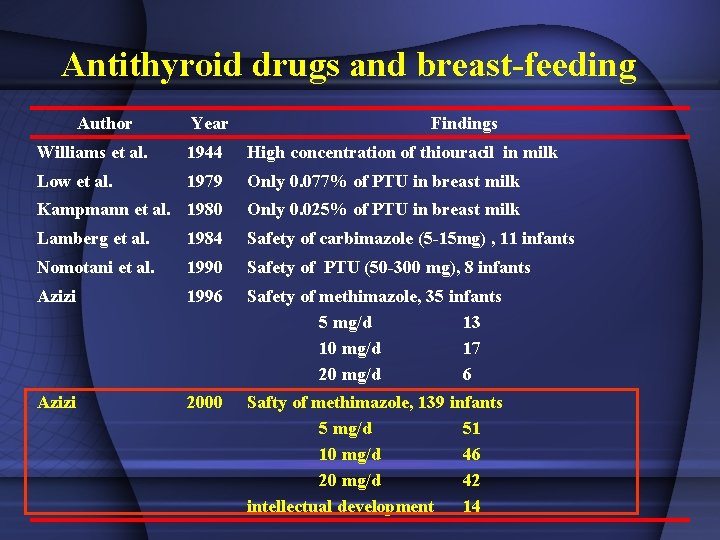
Antithyroid drugs and breast-feeding Author Year Findings Williams et al. 1944 High concentration of thiouracil in milk Low et al. 1979 Only 0. 077% of PTU in breast milk Kampmann et al. 1980 Only 0. 025% of PTU in breast milk Lamberg et al. 1984 Safety of carbimazole (5 -15 mg) , 11 infants Nomotani et al. 1990 Safety of PTU (50 -300 mg), 8 infants Azizi 1996 Safety of methimazole, 35 infants 5 mg/d 13 10 mg/d 17 20 mg/d 6 Azizi 2000 Safty of methimazole, 139 infants 5 mg/d 51 10 mg/d 46 20 mg/d 42 intellectual development 14

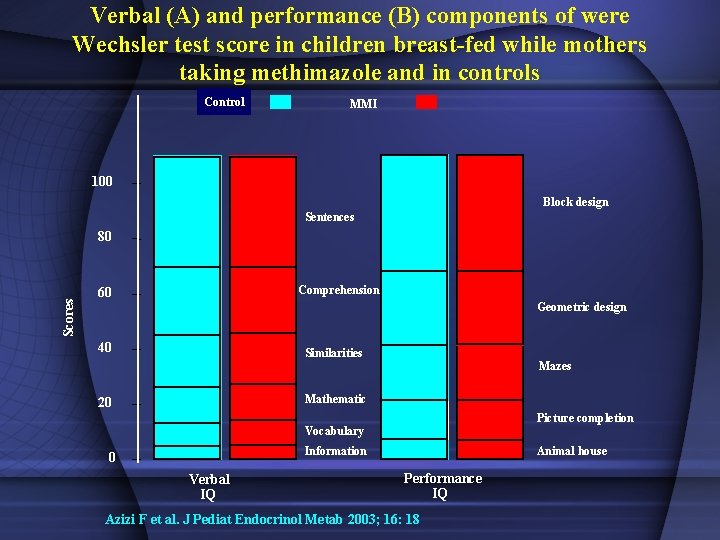
Verbal (A) and performance (B) components of were Wechsler test score in children breast-fed while mothers taking methimazole and in controls Control MMI 100 Block design Sentences Scores 80 Comprehension 60 Geometric design 40 Similarities Mazes Mathematic 20 Picture completion Vocabulary Information 0 Verbal IQ Animal house Performance IQ Azizi F et al. J Pediat Endocrinol Metab 2003; 16: 18

Clinicians should carefully observe the diagnosis, treatment, and follow-up of patients with hyperthyroidism in the pregnancy and postpartum periods. The timely and accurate diagnosis and management are important and improve the quality of life of mothers, fetuses and infants. Thionamide medications remain the mainstay of treatment of hyperthyroidism during pregnancy postpartum and lactation periods. and the

History of Radioiodine Therapy (1) In November 1936, Saul Hertz, the director of the Thyroid Clinic (1931 – 1943) at Massachusetts General Hospital (MGH) in Boston, sat across town in a luncheon meeting at Harvard Medical School, listening to Massachusetts Institute of Technology (MIT) president Karl Compton describe “What Physics Can Do for Biology and Medicine. ” Hertz, who had been studying iodine’s effects on the thyroid, spontaneously asked Compton whether iodine could be made radioactive, his goal being to use this theoretical radioactive iodine to treat hyperthyroidism. Compton responded about a month later by letter, writing that iodine could indeed be made radioactive. Bagley D. Endocrine News 2016; 27 -30

History of Radioiodine Therapy (2) by 1938 Hertz and Roberts began conducting studies with iodine 128 (I-128) on rabbits, showing that the rabbits’ thyroid glands rapidly took up I-128, and thus a tracer was born. However, as Clark T. Sawin and David V. Becker wrote in their paper “Radioiodine and the Treatment of Hyperthyroidism: The Early History, ” published in the journal Thyroid in 1997, there was no hope of using I-128 as a treatment for hyperthyroidism because of I-128’s 25 -minute half-life. Bagley D. Endocrine News 2016; 27 -30

History of Radioiodine Therapy (3) In 1941 the Boston group (with their brand-new cyclotron) was able to create these new radioactive iodines and used a mixture of I-130 and I-131 to treat patients with hyperthyroidism. Hertz administered this therapeutic treatment to the first human patient at MGH in January of 1941, making this year the 75 th anniversary of using a radioactive material to treat a patient — the birth of nuclear medicine. Bagley D. Endocrine News 2016; 27 -30

History of Radioiodine Therapy (4) By 1941, Hertz had advanced his research, and he was administering I-130 to patients at MGH to treat hyperthyroid patients, and over time, he gathered a series of almost 30 patients. Meanwhile, Hitler was advancing through Europe, so Hertz joined the Navy and was shipped off to World War II in 1943. Bagley D. Endocrine News 2016; 27 -30

History of Radioiodine Therapy (5) Hertz shipped out, he asked Chapman to continue his research. “But when Hertz came back from the Navy two years later, it looks like he didn’t want to give it up, and they had a lot of arguments, and they had a rift and weren’t really talking to each other. ” Bagley D. Endocrine News 2016; 27 -30

History of Radioiodine Therapy (6) Hertz used lower doses and followed it with stable iodine, while Chapman was more aggressive, using higher doses without stable iodine. Sawin and Becker also write that they disagreed over “who was in charge” and it “led to their falling out. ” Bagley D. Endocrine News 2016; 27 -30

History of Radioiodine Therapy (7) Chapman wrote his paper first, and sent it to the Journal of the American Medical Association (JAMA) to be published. But the paper was too long and was sent back to Chapman for revisions. Hertz got wind of this, and he rushed to finish his paper. “Although the editor of JAMA was puzzled by two papers on the same topic from the same institution, ” Sawin and Becker write, “both papers appeared in the same issue of JAMA on May 11, 1964, and announced the new therapy was effective treatment for hyperthyroidism. ” Bagley D. Endocrine News 2016; 27 -30

History of Radioiodine Therapy (8) Saul Hertz, sadly, had a relatively short career. He died at age 45 and did not have much time to teach students and pass his findings and techniques on to them. Bagley D. Endocrine News 2016; 27 -30

Radioiodine therapy The most obvious objective in the treatment of hyperthyroidism is to render the patient euthyroid off drug therapy. The major drawback of treatment with thionamide drugs for hyperthyroidism is the frequent reappearance of thyrotoxicosis, up to 20 -70% when therapy is discontinued. Therefore, radioiodine is increasingly considered the treatment of choice because of its safety and ease of administration.

Radioactive Iodine Therapy INDICATIONS: • Patient preference • Toxic MNG • Toxic adenoma • Replace of Graves’ disease After antithyroid therapy After surgery SIDE EFFECTS: • Hypothyroidism • Thyroid storm • Transient neck pain • Siailadenitis • Fetal hypothyroidism or malformation (in pregnancy) • Worsening of ophthalmopathy

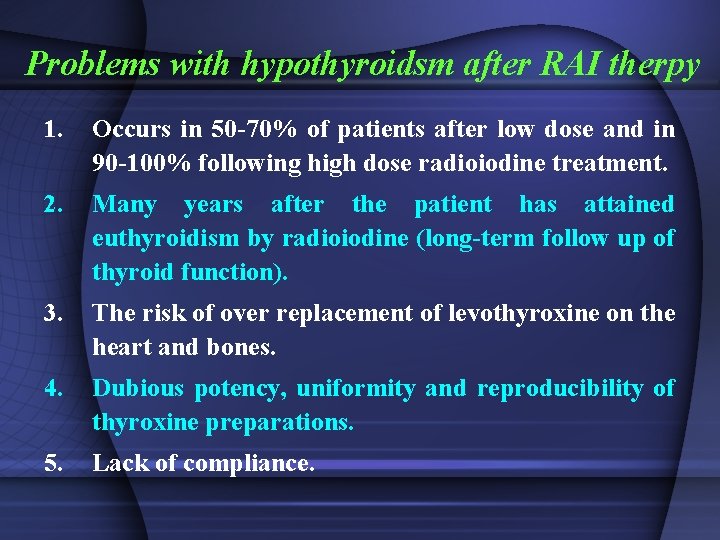
Problems with hypothyroidsm after RAI therpy 1. Occurs in 50 -70% of patients after low dose and in 90 -100% following high dose radioiodine treatment. 2. Many years after the patient has attained euthyroidism by radioiodine (long-term follow up of thyroid function). 3. The risk of over replacement of levothyroxine on the heart and bones. 4. Dubious potency, uniformity and reproducibility of thyroxine preparations. 5. Lack of compliance.

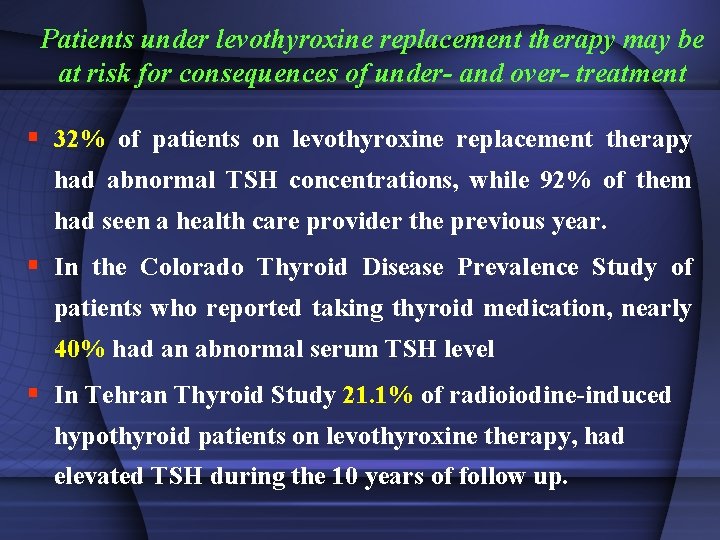
Patients under levothyroxine replacement therapy may be at risk for consequences of under- and over- treatment § 32% of patients on levothyroxine replacement therapy had abnormal TSH concentrations, while 92% of them had seen a health care provider the previous year. § In the Colorado Thyroid Disease Prevalence Study of patients who reported taking thyroid medication, nearly 40% had an abnormal serum TSH level § In Tehran Thyroid Study 21. 1% of radioiodine-induced hypothyroid patients on levothyroxine therapy, had elevated TSH during the 10 years of follow up.

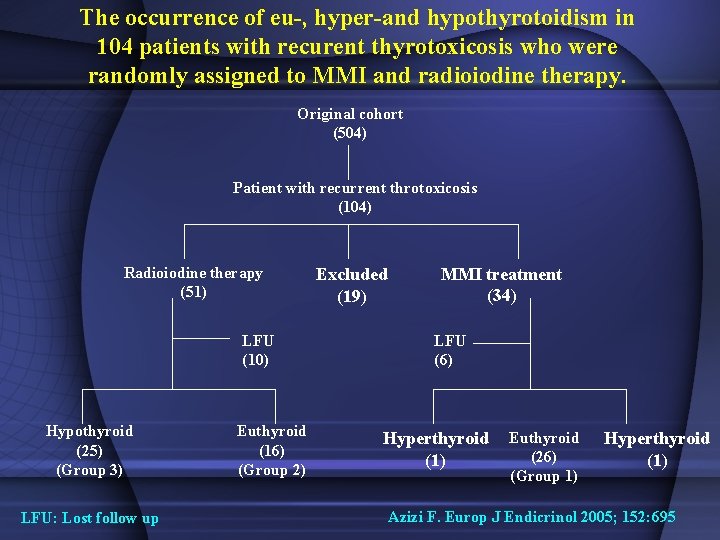
The occurrence of eu-, hyper-and hypothyrotoidism in 104 patients with recurent thyrotoxicosis who were randomly assigned to MMI and radioiodine therapy. Original cohort (504) Patient with recurrent throtoxicosis (104) Radioiodine therapy (51) LFU (10) Hypothyroid (25) (Group 3) LFU: Lost follow up Euthyroid (16) (Group 2) Excluded (19) MMI treatment (34) LFU (6) Hyperthyroid (1) Euthyroid (26) (Group 1) Hyperthyroid (1) Azizi F. Europ J Endicrinol 2005; 152: 695

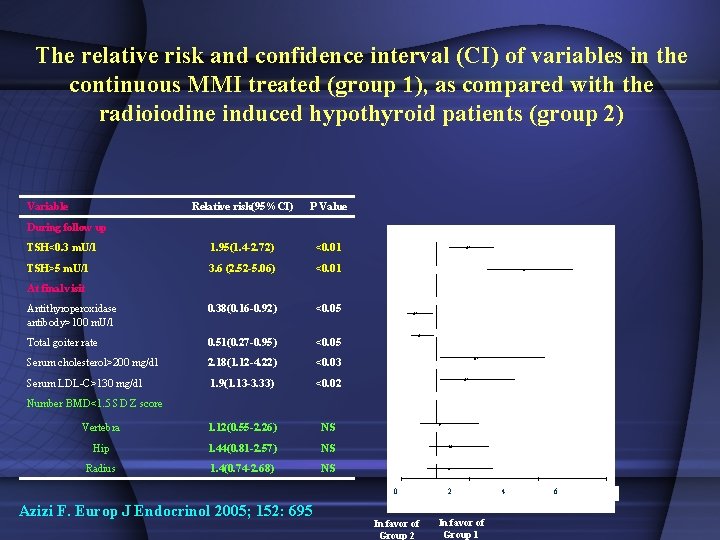
The relative risk and confidence interval (CI) of variables in the continuous MMI treated (group 1), as compared with the radioiodine induced hypothyroid patients (group 2) Variable Relative risk(95%CI) P Value TSH<0. 3 m. U/l 1. 95(1. 4 -2. 72) <0. 01 TSH>5 m. U/l 3. 6 (2. 52 -5. 06) <0. 01 Antithyroperoxidase antibody>100 m. U/l 0. 38(0. 16 -0. 92) <0. 05 Total goiter rate 0. 51(0. 27 -0. 95) <0. 05 Serum cholesterol>200 mg/dl 2. 18(1. 12 -4. 22) <0. 03 Serum LDL-C>130 mg/dl 1. 9(1. 13 -3. 33) <0. 02 Vertebra 1. 12(0. 55 -2. 26) NS Hip 1. 44(0. 81 -2. 57) NS Radius 1. 4(0. 74 -2. 68) NS During follow up At final visit Number BMD<1. 5 SD Z score 0 Azizi F. Europ J Endocrinol 2005; 152: 695 In favor of Group 2 2 In favor of Group 1 4 6

THYROIDECTOMY INDICATIONS: • • • Patient preference Childhood Graves Suggestion of malignancy Sever or advancing ophthalmopathy Young patient with Graves or toxic Adenoma SIDE EFFECTS: • • Hypothyroidism Hypoparathyroidism Recurrent nerve injury Hypocalcemia • Thyroid Storm Anestethic complication Infection Hemorrhage Recurrent thyrotoxicosis Local damage

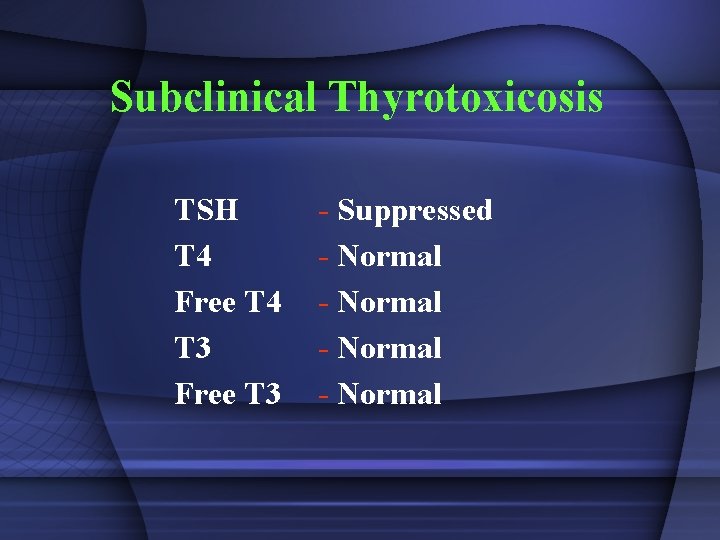
Subclinical Thyrotoxicosis TSH T 4 Free T 4 T 3 Free T 3 - Suppressed - Normal

SUBCLINICAL THYROTOXICOSIS • Decreased thyrotropin, normal L-thyroxine and triiodothyronine and no complaints; • Exogenously caused by L-thyroxine treatment • Endogenous prevalence up to 20% in multinodular goiter • Increased risk for atrial fibrillation, slightly increased bone turnover and decreased bone mineral density in postmenopausal women • Antithyroid drug treatment is indicated in endogenous subclinical thyrotoxicosis associated with atrial fibrillation • A trial of antithyroid drug therapy in asymptomatic patients remains debatable.

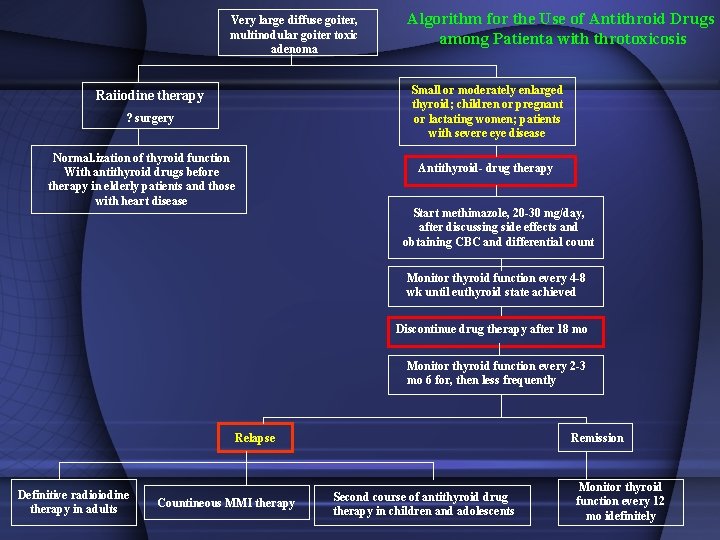
Very large diffuse goiter, multinodular goiter toxic adenoma Algorithm for the Use of Antithroid Drugs among Patienta with throtoxicosis Small or moderately enlarged thyroid; children or pregnant or lactating women; patients with severe eye disease Raiiodine therapy ? surgery Normal. ization of thyroid function With antithyroid drugs before therapy in elderly patients and those with heart disease Antithyroid- drug therapy Start methimazole, 20 -30 mg/day, after discussing side effects and obtaining CBC and differential count Monitor thyroid function every 4 -8 wk until euthyroid state achieved Discontinue drug therapy after 18 mo Monitor thyroid function every 2 -3 mo 6 for, then less frequently Relapse Definitive radioiodine therapy in adults Countineous MMI therapy Remission Second course of antithyroid drug therapy in children and adolescents Monitor thyroid function every 12 mo idefinitely

 Fereidoun azizi
Fereidoun azizi Tiroid storm
Tiroid storm Hyperthyroidism treatment
Hyperthyroidism treatment Sadoon azizi
Sadoon azizi Sadoon azizi
Sadoon azizi Myxedema coma signs and symptoms
Myxedema coma signs and symptoms Levothyroxine side effects
Levothyroxine side effects Subclinical hyperthyroidism
Subclinical hyperthyroidism Type of hyperthyroidism
Type of hyperthyroidism Hyperthyroidism primary and secondary
Hyperthyroidism primary and secondary Myxedema
Myxedema Tsh levels low
Tsh levels low Graves desease
Graves desease Thyroid scintigraphy
Thyroid scintigraphy Primary vs secondary hypothyroidism
Primary vs secondary hypothyroidism Thyroid
Thyroid Hyperthyroidism in babies
Hyperthyroidism in babies Thyroid effects in females
Thyroid effects in females Hypothyroidism
Hypothyroidism Hyperthyroidism
Hyperthyroidism Cupping and autoimmune disease
Cupping and autoimmune disease Ucla autism research
Ucla autism research Research report vs research proposal
Research report vs research proposal Method vs methodology
Method vs methodology Appendices in research example
Appendices in research example Conclusive vs exploratory research
Conclusive vs exploratory research What is applied research
What is applied research What is research gap meaning
What is research gap meaning Contrast applied research and basic research
Contrast applied research and basic research Research problem definition
Research problem definition Research paradigm example
Research paradigm example Operational thought
Operational thought Causal comparative research definition
Causal comparative research definition Chapter 3 methodology
Chapter 3 methodology The difference between basic research and applied research
The difference between basic research and applied research Basic research vs applied research
Basic research vs applied research Objectives of reseach
Objectives of reseach Qualitative research research design
Qualitative research research design Practical research 2 nature of inquiry and research
Practical research 2 nature of inquiry and research Characteristics of conclusive research
Characteristics of conclusive research Research design exploratory
Research design exploratory Purpose of the study in research proposal example
Purpose of the study in research proposal example Research instrument in experimental research
Research instrument in experimental research Eight characteristics educational research
Eight characteristics educational research Water treatment fundamentals
Water treatment fundamentals Tertiary treatment of wastewater apes
Tertiary treatment of wastewater apes Wastewater treatment purpose
Wastewater treatment purpose