Topic 8 3 Stoichiometry Limiting and Excess Reagent



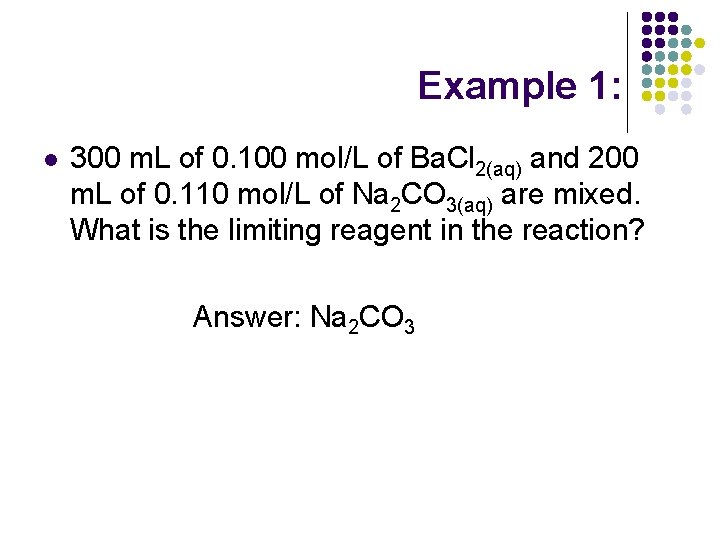







- Slides: 22

Topic 8. 3 Stoichiometry: Limiting and Excess Reagent Calculations Page 320 -327 By Kirsten

What is Stoichiometry? Lets take a closer look. l l A method of predicting or analyzing the quantities of the reactants and products participating in a chemical process Three forms including gas stoichiometry, solution stoichiometry, and gravimetric stoichiometry.

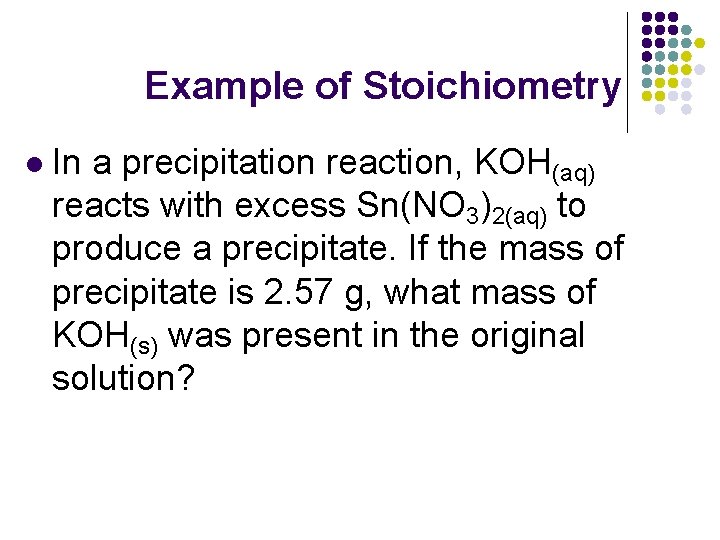
Example of Stoichiometry l In a precipitation reaction, KOH(aq) reacts with excess Sn(NO 3)2(aq) to produce a precipitate. If the mass of precipitate is 2. 57 g, what mass of KOH(s) was present in the original solution?

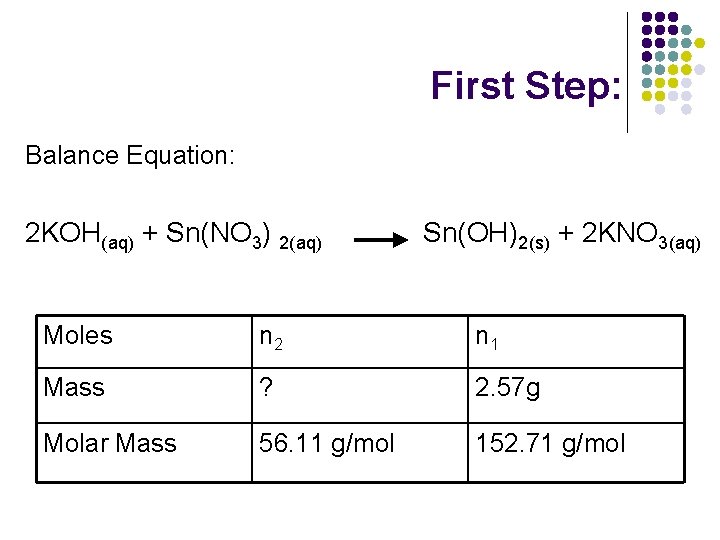
First Step: Balance Equation: 2 KOH(aq) + Sn(NO 3) 2(aq) Sn(OH)2(s) + 2 KNO 3(aq) Moles n 2 n 1 Mass ? 2. 57 g Molar Mass 56. 11 g/mol 152. 71 g/mol

Second Step: n 1= 2. 57 g x (1 mol/ 152. 71 g) = 0. 0168 mol n 2= 0. 0168 mol x (2/1) = 0. 0337 mol m= 0. 0337 mol x (56. 11 mol/g) = 1. 89 g

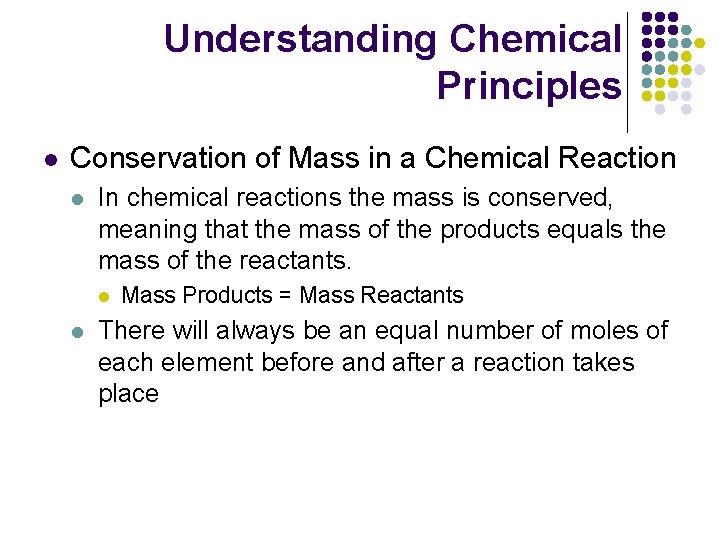
Understanding Chemical Principles l Conservation of Mass in a Chemical Reaction l In chemical reactions the mass is conserved, meaning that the mass of the products equals the mass of the reactants. l l Mass Products = Mass Reactants There will always be an equal number of moles of each element before and after a reaction takes place

Identifying Reagents l Limiting Reagents l l Excess Reagents l l Completely consumed in a chemical reaction More is present than is necessary to react with the limiting reagent The limited reagent is the reagent that is being analyzed in a quantitative analysis where limiting and excess reagents are present.

How to find Limiting Regent: l l l Ensure that all masses given are in moles. Take the molar amounts from each substance and divide by the coefficient of that substance in the balanced equation. The smaller number will be the limiting reagent.
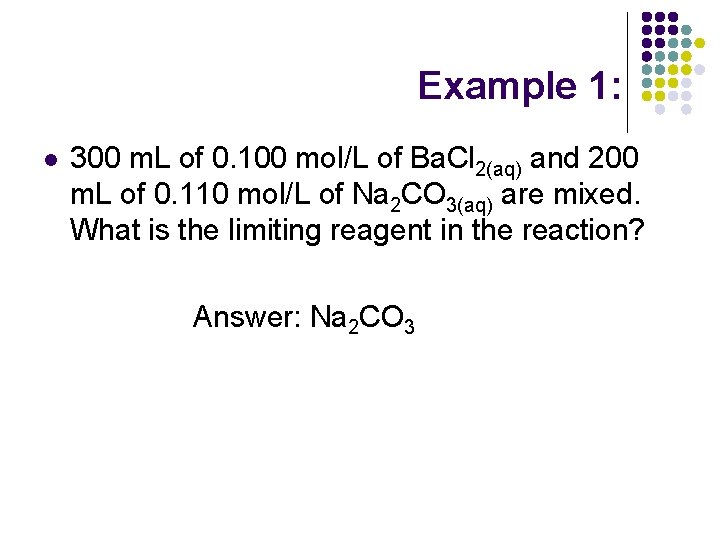
Example 1: l 300 m. L of 0. 100 mol/L of Ba. Cl 2(aq) and 200 m. L of 0. 110 mol/L of Na 2 CO 3(aq) are mixed. What is the limiting reagent in the reaction? Answer: Na 2 CO 3

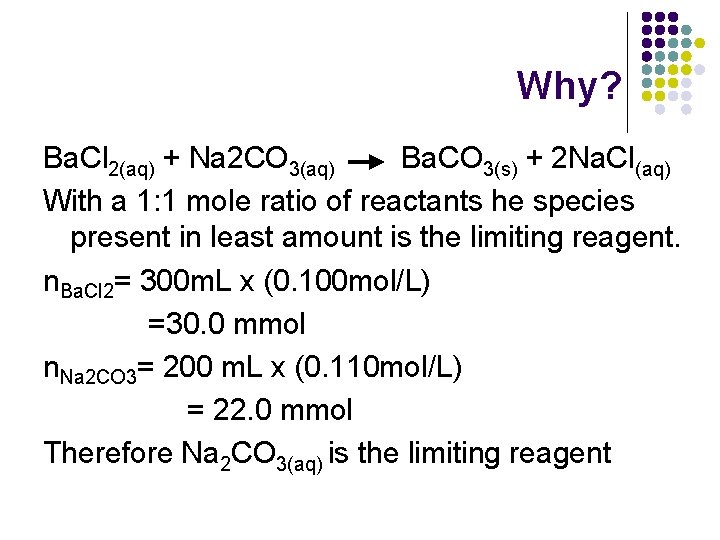
Why? Ba. Cl 2(aq) + Na 2 CO 3(aq) Ba. CO 3(s) + 2 Na. Cl(aq) With a 1: 1 mole ratio of reactants he species present in least amount is the limiting reagent. n. Ba. Cl 2= 300 m. L x (0. 100 mol/L) =30. 0 mmol n. Na 2 CO 3= 200 m. L x (0. 110 mol/L) = 22. 0 mmol Therefore Na 2 CO 3(aq) is the limiting reagent

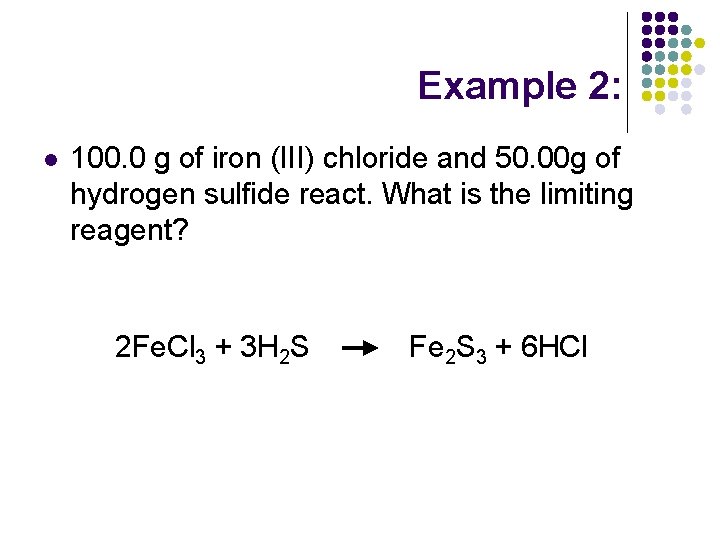
Example 2: l 100. 0 g of iron (III) chloride and 50. 00 g of hydrogen sulfide react. What is the limiting reagent? 2 Fe. Cl 3 + 3 H 2 S Fe 2 S 3 + 6 HCl

Answer: l l l Iron(III) chloride 100 g/ 162. 204 g/mol =0. 6165 mol Hydrogen Sulfide 50. 00 g/ 34. 081 g/mol =1. 467 mol/ 3 =0. 489 Iron (III) chloride is the limiting reagent

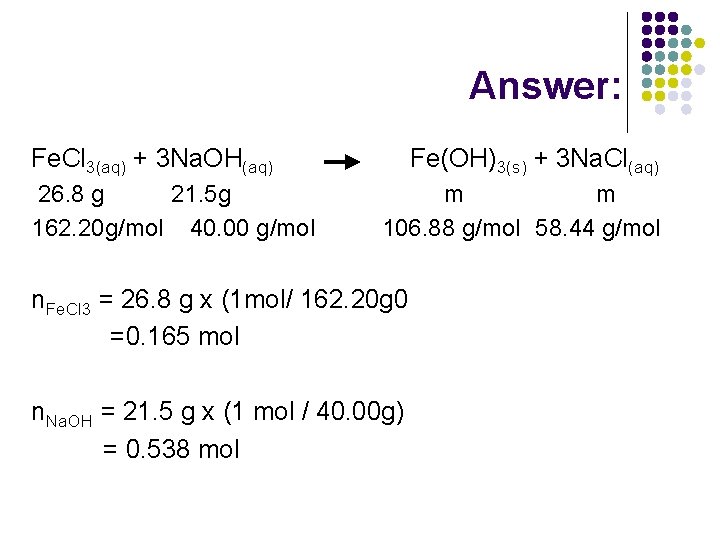
Example 3: l In an experiment, 26. 8 g of iron(III) chloride in solution is combined with 21. 5 g of sodium hydroxide in solution. Which reactant is in excess, and by how much? What mass of each product will be obtained?

Answer: Fe. Cl 3(aq) + 3 Na. OH(aq) 26. 8 g 21. 5 g 162. 20 g/mol 40. 00 g/mol Fe(OH)3(s) + 3 Na. Cl(aq) m m 106. 88 g/mol 58. 44 g/mol n. Fe. Cl 3 = 26. 8 g x (1 mol/ 162. 20 g 0 =0. 165 mol n. Na. OH = 21. 5 g x (1 mol / 40. 00 g) = 0. 538 mol

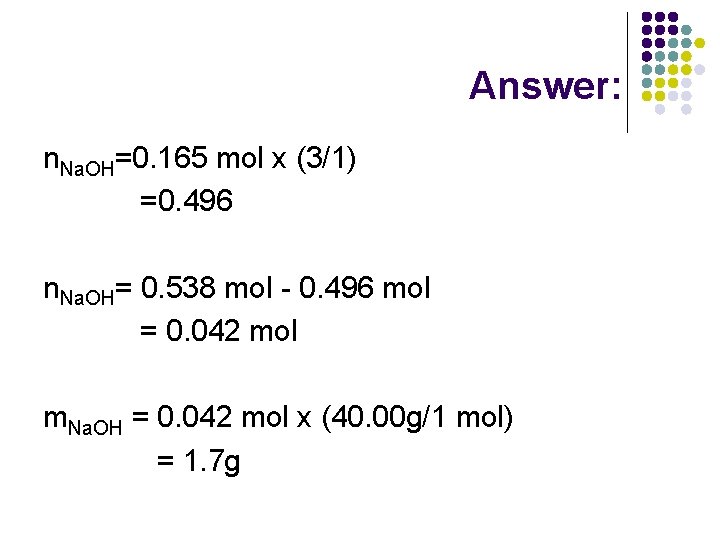
Answer: n. Na. OH=0. 165 mol x (3/1) =0. 496 n. Na. OH= 0. 538 mol - 0. 496 mol = 0. 042 mol m. Na. OH = 0. 042 mol x (40. 00 g/1 mol) = 1. 7 g

Answer: m. Fe(OH)3= 0. 165 mol. Fe. Cl 3 x (1 mol Fe(OH)3/1 mol. Fe. Cl 3) x (106. 88 g. Fe(OH)3/ 1 mol Fe(OH)3) = 17. 7 g Fe(OH)3 m Na. Cl = 0. 165 mol. Fe. Cl 3 x (3 mol Na. Cl/ 1 mol Fe. Cl 3) x (58. 44 g Na. Cl / 1 mol Na. Cl) = 29. 0 Na. Cl Therefore sodium hydroxide is in excess by 1. 7 g, the mass of iron (III) hydroxide produced is 17. 7 g and the mass of sodium chloride produced is 29. 0 g.

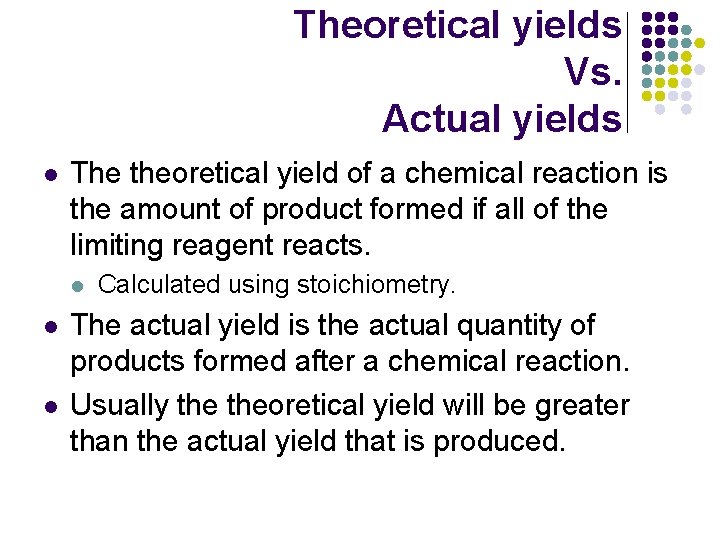
Theoretical yields Vs. Actual yields l The theoretical yield of a chemical reaction is the amount of product formed if all of the limiting reagent reacts. l l l Calculated using stoichiometry. The actual yield is the actual quantity of products formed after a chemical reaction. Usually theoretical yield will be greater than the actual yield that is produced.

Reasons for Discrepancy l The actual yield of a chemical reaction is usually less than theoretical yield for these reasons l l l Purity of chemicals being used Errors in measurements Experimental factors that may have lead to loss of reactants

% Error Calculations: l A reasonable quantity of reasonable excess reagent is 10%

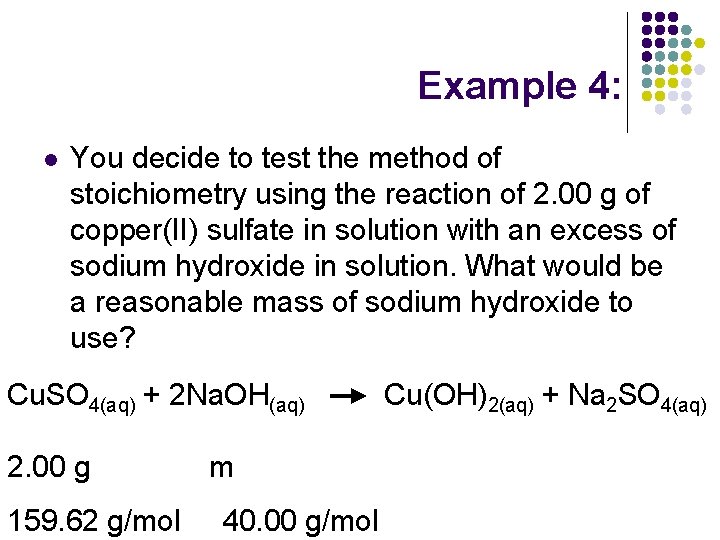
Example 4: l You decide to test the method of stoichiometry using the reaction of 2. 00 g of copper(II) sulfate in solution with an excess of sodium hydroxide in solution. What would be a reasonable mass of sodium hydroxide to use? Cu. SO 4(aq) + 2 Na. OH(aq) 2. 00 g 159. 62 g/mol m 40. 00 g/mol Cu(OH)2(aq) + Na 2 SO 4(aq)

Answer: n. Cu. SO 4 = 2. 00 g x (1 mol/159. 62 g) =0. 0125 mol n. Na. OH =0. 0125 mol x (2/1) =0. 0251 mol m. Na. OH = 0. 0251 mol x (40 g/1 mol) = 1. 00 g Now add 10% to this amount to determine the reasonable mass of sodium hydroxide to use. 1. 00 g + 0. 10 g =1. 10 g

For More Information: l Nelson Chemistry Text l l l Pages 320 -327 The Key- Chemistry 20 D 2 L Web Lessons