Lesson 11 Stoichiometry 11 1 How Much 11










- Slides: 27

Lesson 11 Stoichiometry 11. 1 – How Much? ? 11. 2 – Mole Ratios 11. 3 – Excess and Limiting Reactants 11. 4 – Percent Yield 11. 5 – Molarity Anything in black letters = write it in your notes (‘knowts’)

11. 1 – How Much? ? Stoichiometry Quantitative study of chemical rxns. In this lesson we will be asking ‘how much? ’

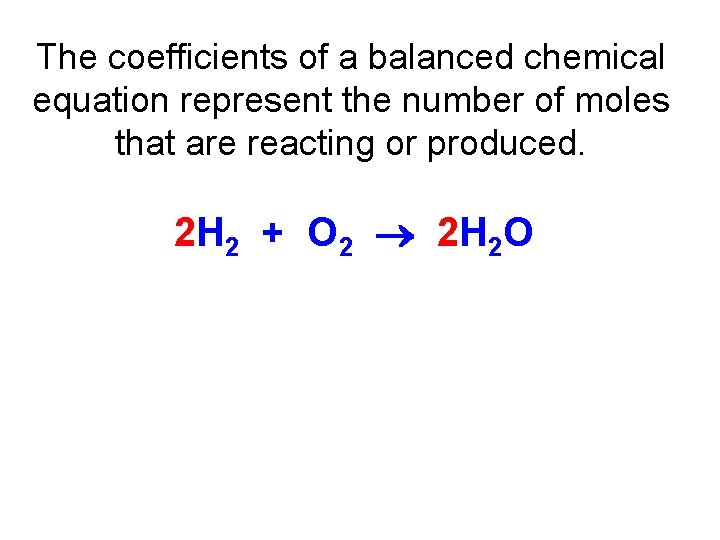
The coefficients of a balanced chemical equation represent the number of moles that are reacting or produced. 2 H 2 + O 2 2 H 2 O

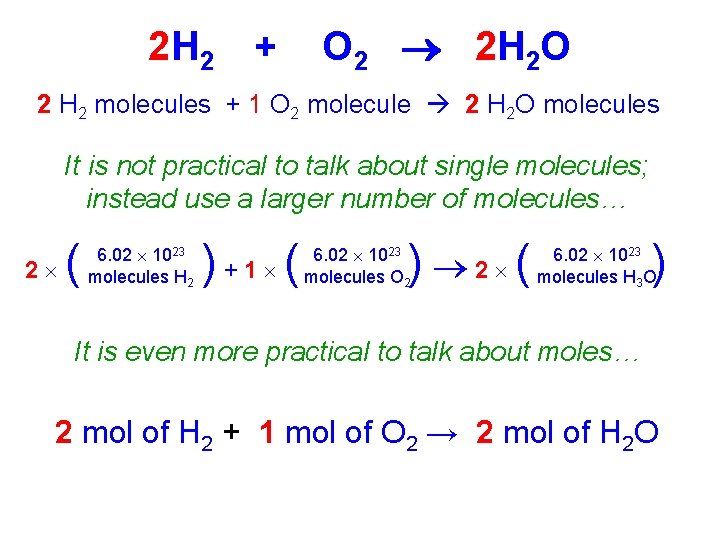
2 H 2 + O 2 2 H 2 O 2 H 2 molecules + 1 O 2 molecule 2 H 2 O molecules It is not practical to talk about single molecules; instead use a larger number of molecules… 2 ( 6. 02 1023 molecules H 2 )+1 ( ) 2 ( 6. 02 1023 molecules O 2 ) 6. 02 1023 molecules H 3 O It is even more practical to talk about moles… 2 mol of H 2 + 1 mol of O 2 → 2 mol of H 2 O

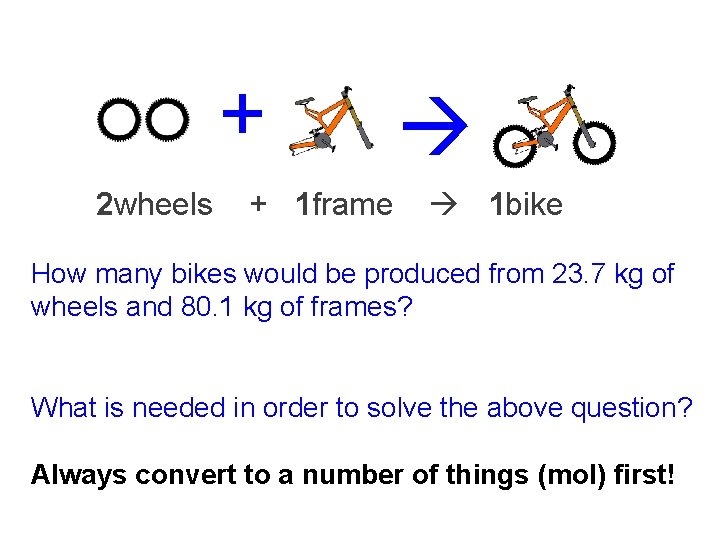
The bike example… For simplicity, say a bike requires only 1 frame and 2 wheels. 2 wheels + 1 frame 1 bike What are the coefficients here? What do they tell you?

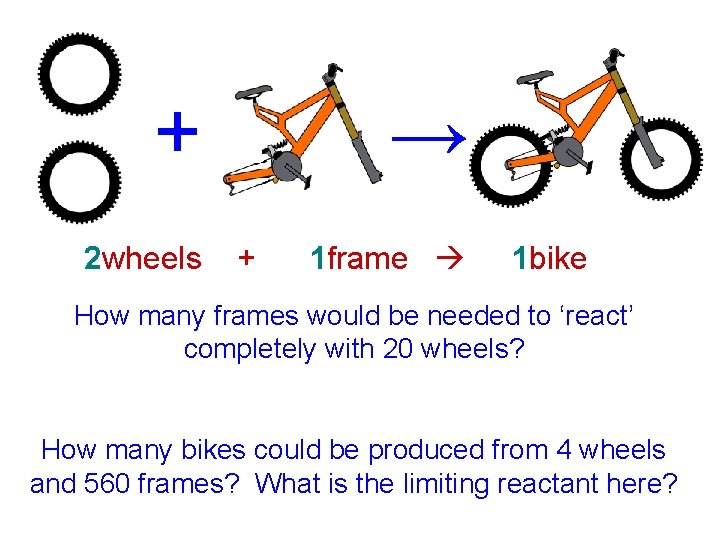
+ 2 wheels → + 1 frame 1 bike How many frames would be needed to ‘react’ completely with 20 wheels? How many bikes could be produced from 4 wheels and 560 frames? What is the limiting reactant here?

+ 2 wheels + 1 frame 1 bike How many bikes would be produced from 23. 7 kg of wheels and 80. 1 kg of frames? What is needed in order to solve the above question? Always convert to a number of things (mol) first!

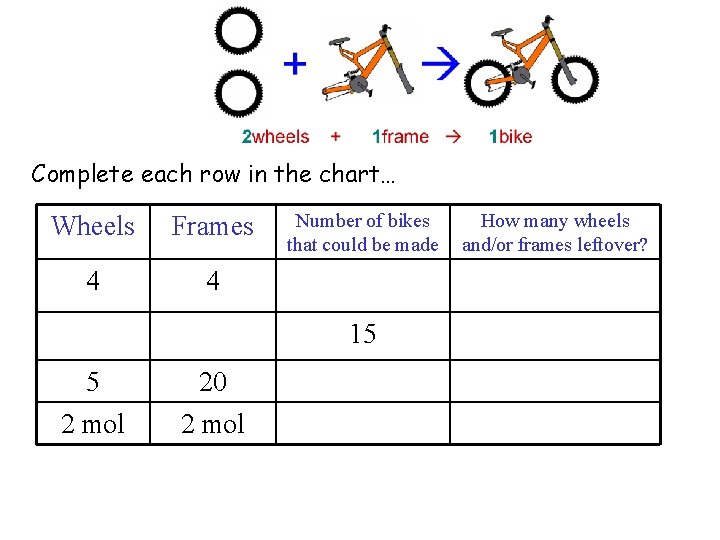
Complete each row in the chart… Wheels Frames 4 4 Number of bikes that could be made 15 5 2 mol 20 2 mol How many wheels and/or frames leftover?

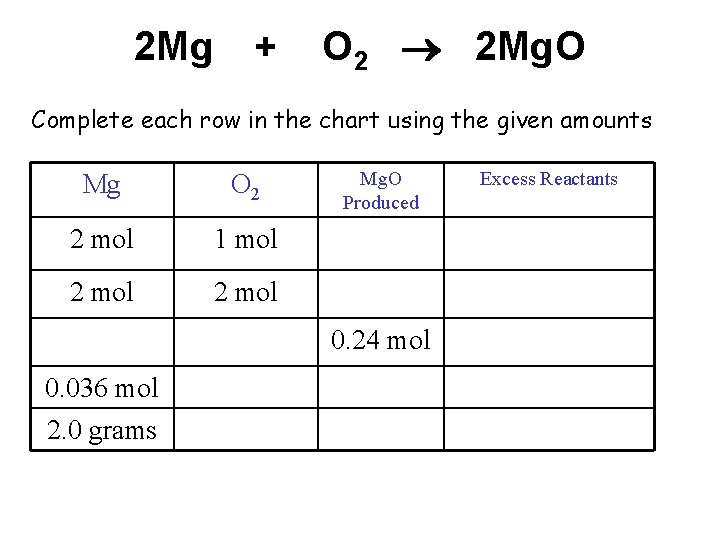
2 Mg + O 2 2 Mg. O Complete each row in the chart using the given amounts Mg O 2 2 mol 1 mol 2 mol Mg. O Produced 0. 24 mol 0. 036 mol 2. 0 grams Excess Reactants

11. 2 – Mole Ratios A mole ratio comes from the coefficients of a balanced chemical equation. Mole ratios are used to compare the amount of mol of one substance to another.

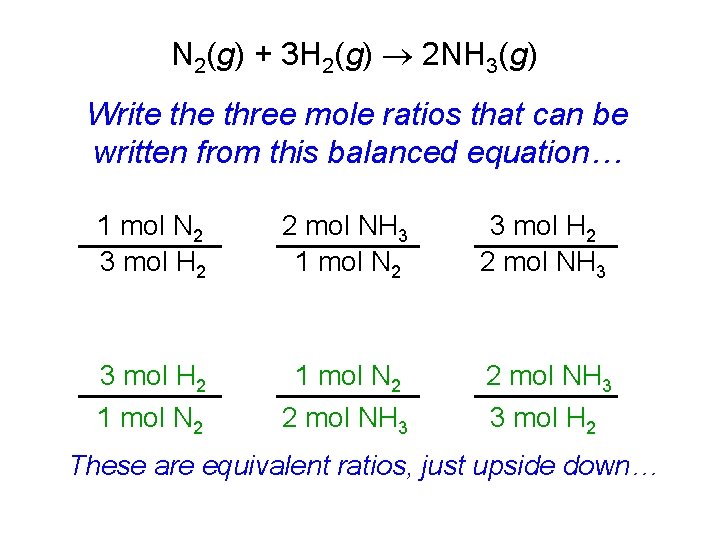
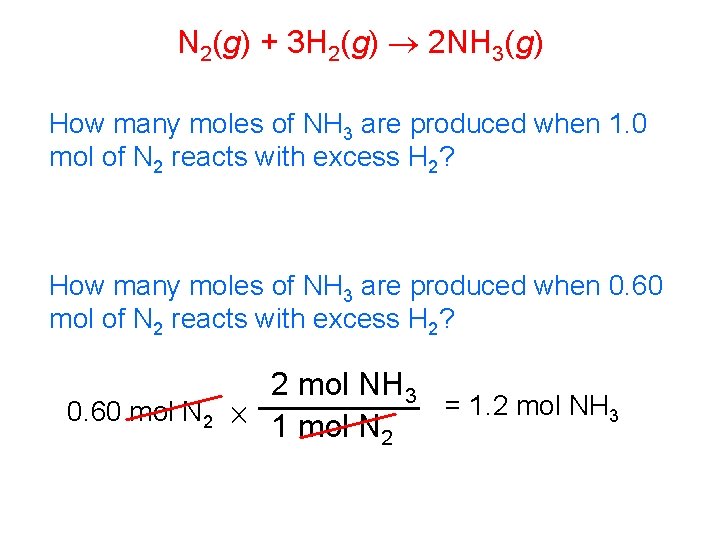
N 2(g) + 3 H 2(g) 2 NH 3(g) Write three mole ratios that can be written from this balanced equation… 1 mol N 2 3 mol H 2 2 mol NH 3 3 mol H 2 1 mol N 2 2 mol NH 3 3 mol H 2 These are equivalent ratios, just upside down…

N 2(g) + 3 H 2(g) 2 NH 3(g) How many moles of NH 3 are produced when 1. 0 mol of N 2 reacts with excess H 2? How many moles of NH 3 are produced when 0. 60 mol of N 2 reacts with excess H 2? 2 mol NH 3 = 1. 2 mol NH 3 0. 60 mol N 2 1 mol N 2

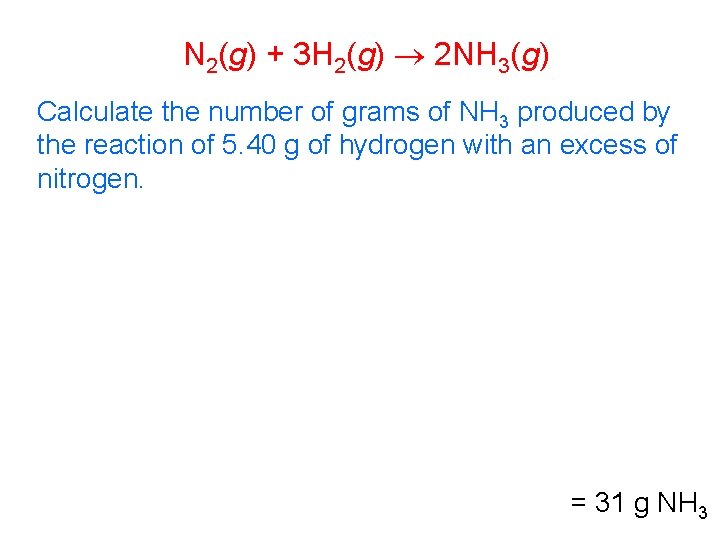
N 2(g) + 3 H 2(g) 2 NH 3(g) Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. = 31 g NH 3

4 P(s) + 5 O 2(g) P 4 O 10(s) What mass of phosphorus will be needed to produce 3. 25 mol of P 4 O 10? = 403 g P

How to Solve Stoichiometric Problems Streamlined 1. Convert given # into moles, if it isn’t already 2. Multiply by the mole ratio conversion factor 3. Convert from moles of substance into desired unit if necessary.

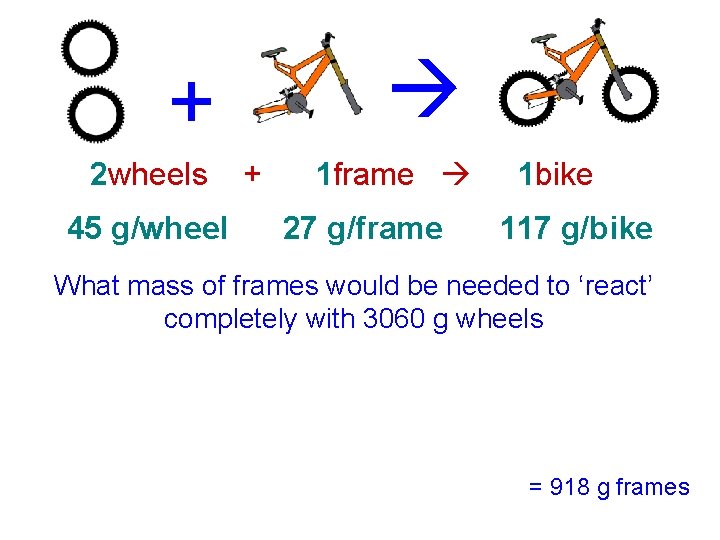
+ 2 wheels 45 g/wheel + 1 frame 27 g/frame 1 bike 117 g/bike What mass of frames would be needed to ‘react’ completely with 3060 g wheels = 918 g frames

11. 3 – Excess and Limiting Reactants The substance that is completely used up in a chemical rxn is called the limiting reactant. The substance that is NOT completely used up (and partially remains) is the excess reactant.

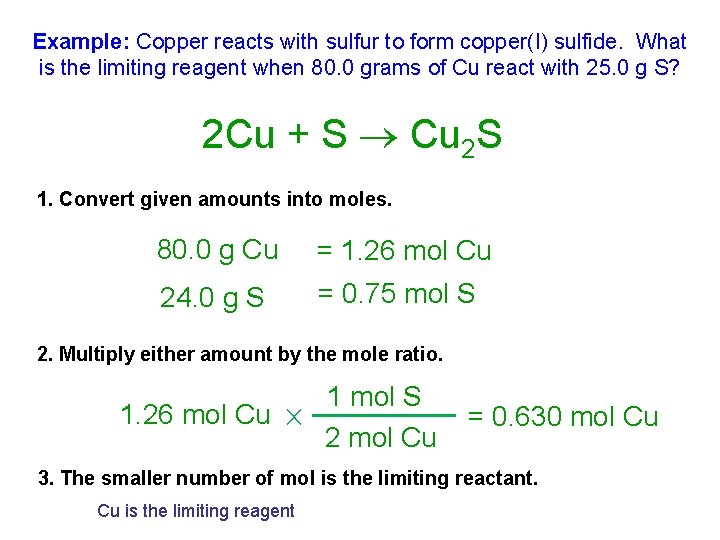
Example: Copper reacts with sulfur to form copper(I) sulfide. What is the limiting reagent when 80. 0 grams of Cu react with 25. 0 g S? 2 Cu + S Cu 2 S 1. Convert given amounts into moles. 80. 0 g Cu = 1. 26 mol Cu 24. 0 g S = 0. 75 mol S 2. Multiply either amount by the mole ratio. 1. 26 mol Cu 1 mol S 2 mol Cu = 0. 630 mol Cu 3. The smaller number of mol is the limiting reactant. Cu is the limiting reagent

You Try It! 2 Fe + O 2 + 2 H 2 O 2 Fe(OH)2 If 7. 0 g of iron and 9. 0 g of water are available to react, which is the limiting reagent? 1. Calculate the amount of one reactant required to react with the other. 2. Compare the given amount to the required amount.

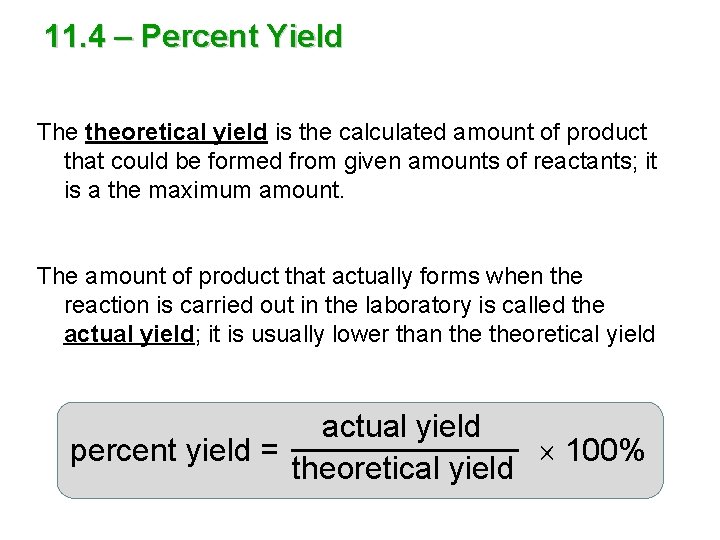
11. 4 – Percent Yield The theoretical yield is the calculated amount of product that could be formed from given amounts of reactants; it is a the maximum amount. The amount of product that actually forms when the reaction is carried out in the laboratory is called the actual yield; it is usually lower than theoretical yield actual yield percent yield = 100% theoretical yield

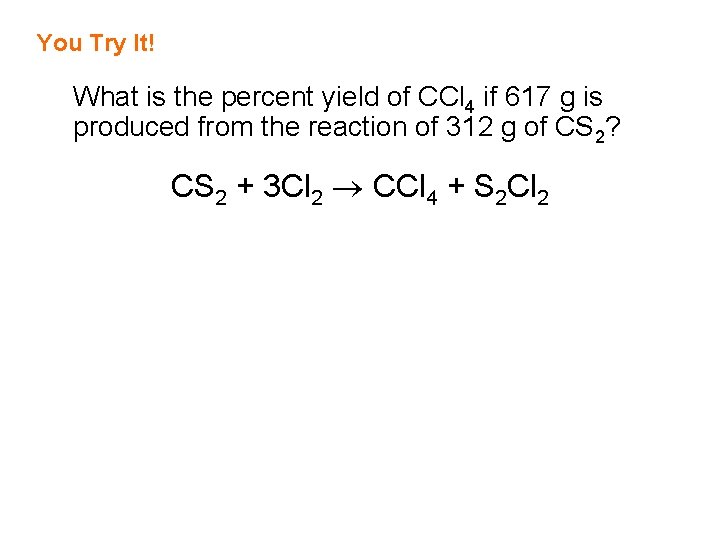
You Try It! What is the percent yield of CCl 4 if 617 g is produced from the reaction of 312 g of CS 2? CS 2 + 3 Cl 2 CCl 4 + S 2 Cl 2

Practice Quiz Practice Learn from mistakes Repeat Check answer key Use class time for work time and help Quiz “Studying” is a myth Best way to study is to re-do practice quiz Some points can be recovered in 1: 1 time with Tischer

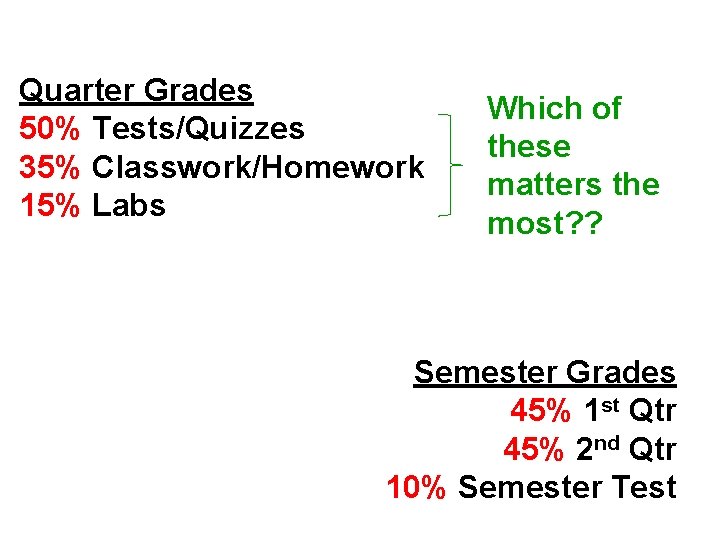
Quarter Grades 50% Tests/Quizzes 35% Classwork/Homework 15% Labs Which of these matters the most? ? Semester Grades 45% 1 st Qtr 45% 2 nd Qtr 10% Semester Test

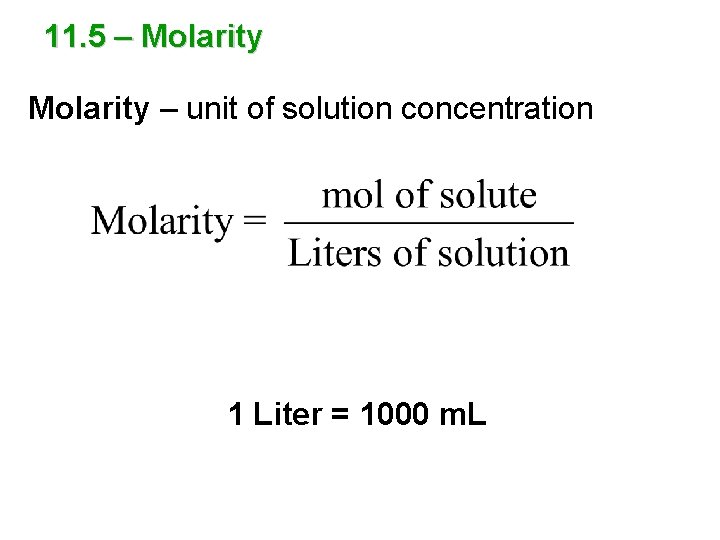
11. 5 – Molarity – unit of solution concentration 1 Liter = 1000 m. L

Calculate the molarity of these solutions. a) 0. 55 mol Na. OH dissolved in 1. 0 L solution b) 4. 0 grams of Na. OH dissolved in 1. 0 L solution c) 4. 0 grams of Na. OH dissolved in 250 m. L solution

How many moles of solute would be in the following solutions? a) 1. 00 Liter of 2. 2 M HNO 3 b) 25. 0 m. L of 0. 225 M HCl

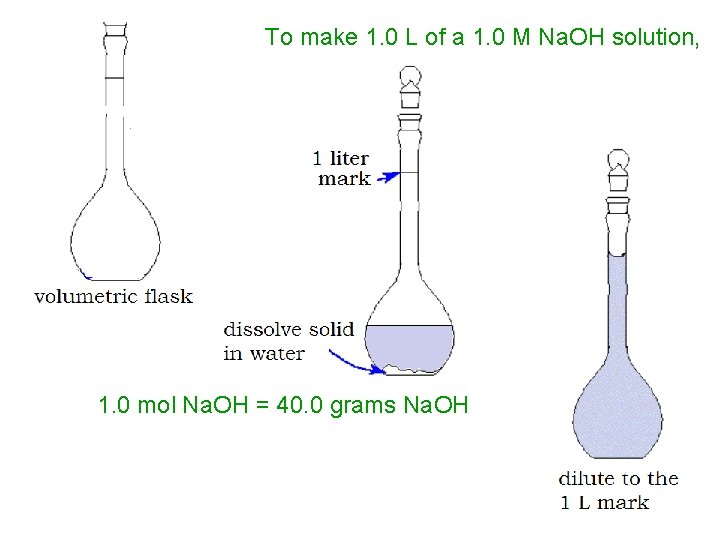
To make 1. 0 L of a 1. 0 M Na. OH solution, 1. 0 mol Na. OH = 40. 0 grams Na. OH