Limiting Reagents Limiting Reagent If there isnt enough





- Slides: 10

Limiting Reagents

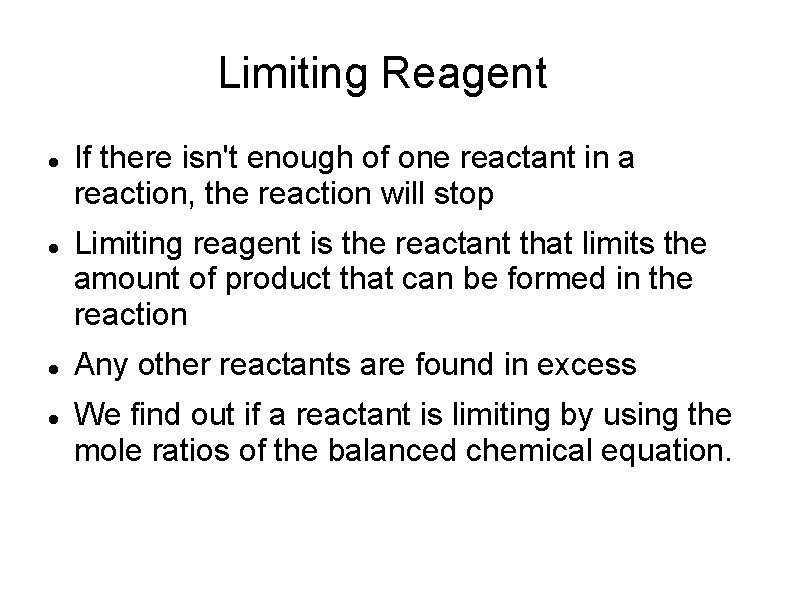
Limiting Reagent If there isn't enough of one reactant in a reaction, the reaction will stop Limiting reagent is the reactant that limits the amount of product that can be formed in the reaction Any other reactants are found in excess We find out if a reactant is limiting by using the mole ratios of the balanced chemical equation.

Remember! You CANNOT compare masses of reactants and products To find out if a reactant is limiting you MUST compare moles!

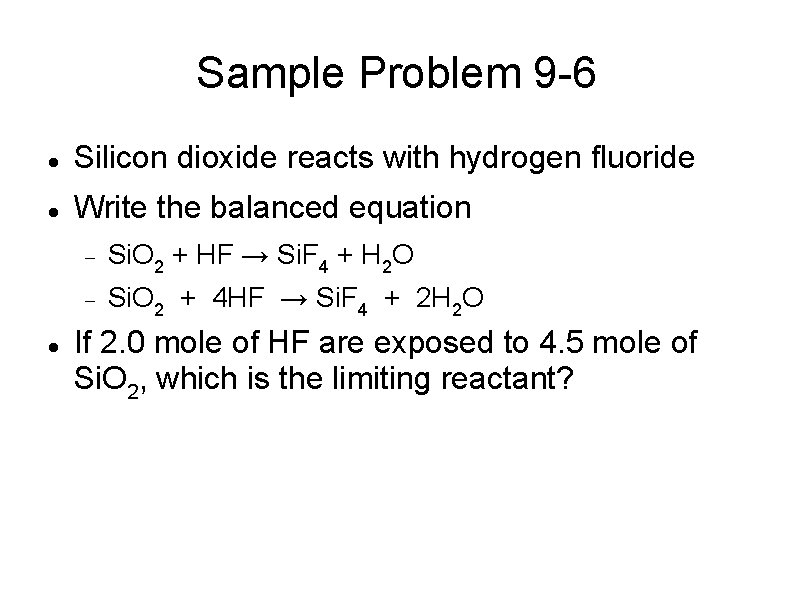
Sample Problem 9 -6 Silicon dioxide reacts with hydrogen fluoride Write the balanced equation Si. O 2 + HF → Si. F 4 + H 2 O Si. O 2 + 4 HF → Si. F 4 + 2 H 2 O If 2. 0 mole of HF are exposed to 4. 5 mole of Si. O 2, which is the limiting reactant?

Choose one of the reactants to begin Use the mole ratio: 2. 0 mole HF x 1 mole Si. O 2 = 0. 50 mole Si. O 2 4 mole HF After 2. 0 mole HF used up, only 0. 50 mole Si. O 2 used. HF is limiting. There is excess Si. O 2

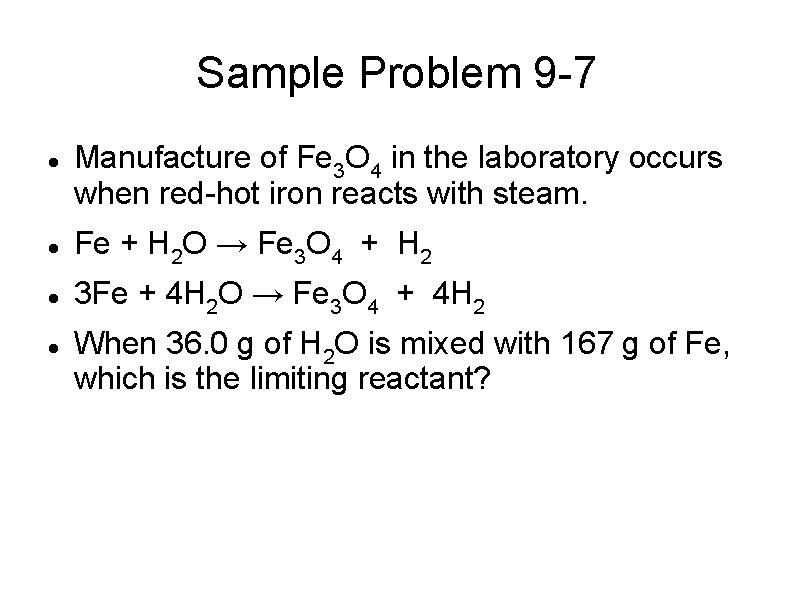
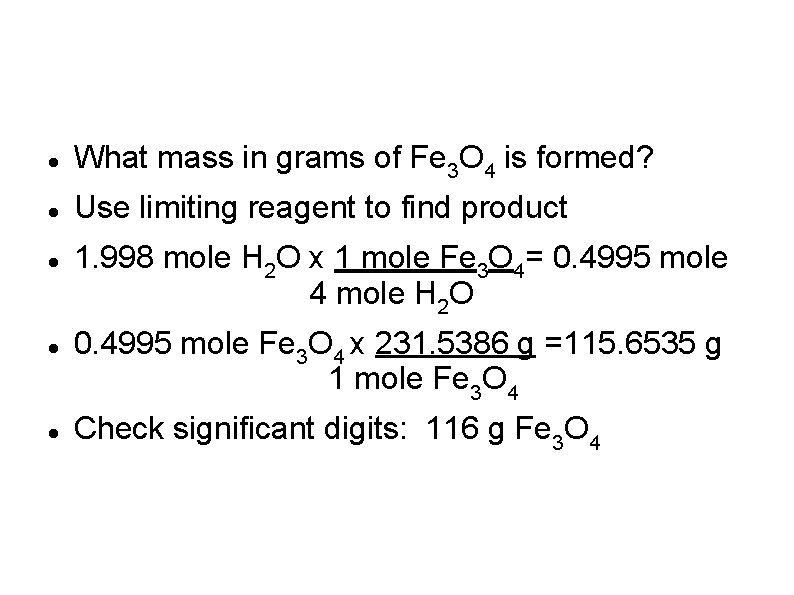
Sample Problem 9 -7 Manufacture of Fe 3 O 4 in the laboratory occurs when red-hot iron reacts with steam. Fe + H 2 O → Fe 3 O 4 + H 2 3 Fe + 4 H 2 O → Fe 3 O 4 + 4 H 2 When 36. 0 g of H 2 O is mixed with 167 g of Fe, which is the limiting reactant?

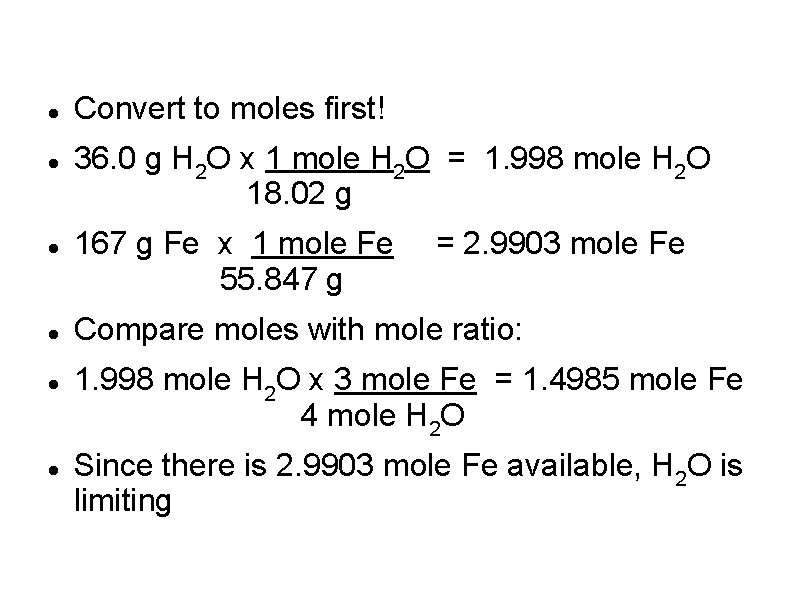
Convert to moles first! 36. 0 g H 2 O x 1 mole H 2 O = 1. 998 mole H 2 O 18. 02 g 167 g Fe x 1 mole Fe 55. 847 g = 2. 9903 mole Fe Compare moles with mole ratio: 1. 998 mole H 2 O x 3 mole Fe = 1. 4985 mole Fe 4 mole H 2 O Since there is 2. 9903 mole Fe available, H 2 O is limiting

What mass in grams of Fe 3 O 4 is formed? Use limiting reagent to find product 1. 998 mole H 2 O x 1 mole Fe 3 O 4= 0. 4995 mole 4 mole H 2 O 0. 4995 mole Fe 3 O 4 x 231. 5386 g =115. 6535 g 1 mole Fe 3 O 4 Check significant digits: 116 g Fe 3 O 4

What mass of the excess reactant remains? Remaining: 2. 9903 - 1. 4985 mole = 1. 4918 mole Fe x 55. 847 g Fe = 83. 31244 g Fe 1 mole Fe Check significant digits! 83. 3 g Fe remains

Now it's your job! After reading pages 288 – 291, do practice problems 1 -2 on page 289 and practice problems 1 -2 page 291