limiting reagents Introduction to limiting reagents a My




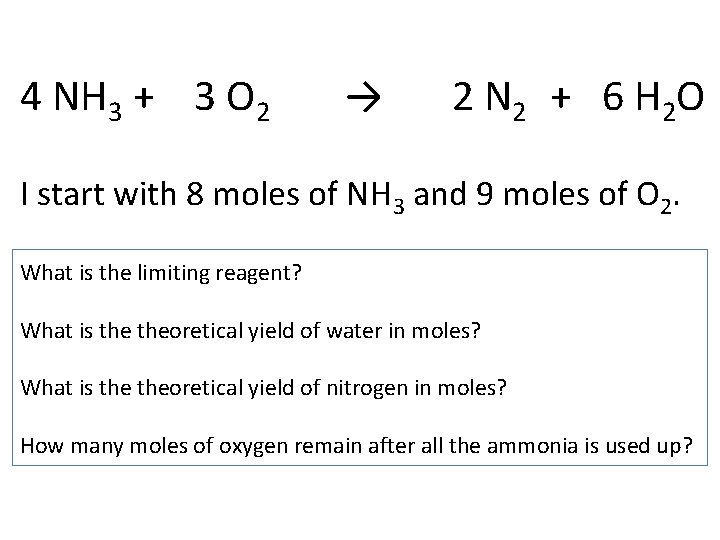
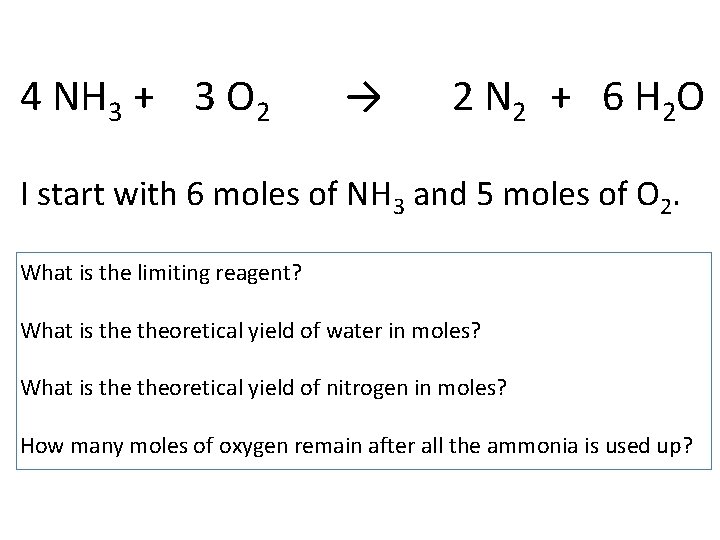
- Slides: 17
limiting reagents

Introduction to limiting reagents a) My goal is to make 5 cheesecakes. How many eggs, blocks of cream cheese and cups of sugar are needed? b) My goal is to make 5 dozen cheesecakes. How many dozens ofeggs, dozens of blocks of cream cheese and dozens of cups of sugar are needed? c) My goal is to make 5 moles of cheesecakes. How many moles of eggs, moles of blocks of cream cheese and moles of cups of sugar are needed?

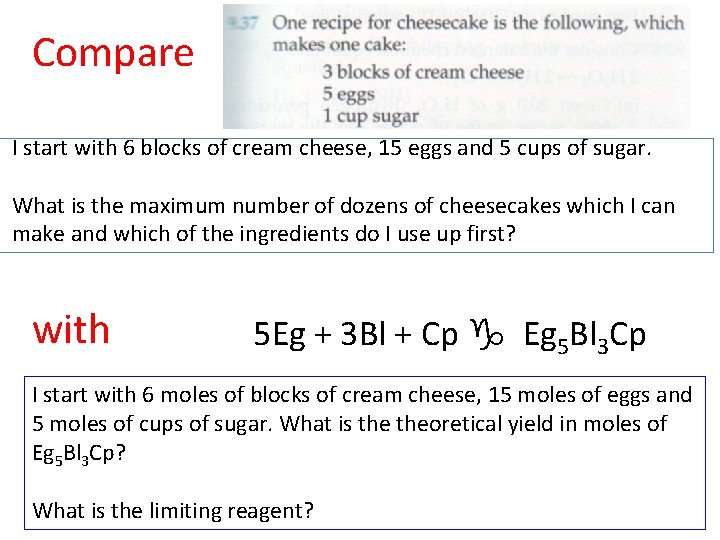
Introduction to limiting reagents I start with 6 blocks of cream cheese, 15 eggs and 5 cups of sugar. What is the maximum number of dozens of cheesecakes which I can make? The theoretical yield is the maximum number of cheesecakes which, under these conditions, can be made. The limiting reagent is the ingredient which gets used up first when cheesecakes are made. What is the limiting reagent in the above situation?

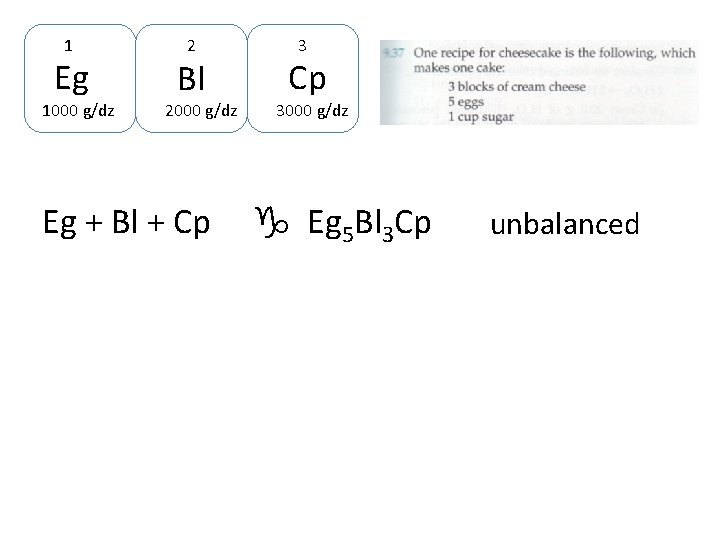
Let’s turn this recipe into chemistry format. 1 dozen Eg are 1000 g 1 Eg 1000 g/dz 1 dz, Bl are 2000 g 2 Bl 2000 g/dz 1 dz Cp = 3000 g 3 Cp 3000 g/dz

Let’s turn this recipe into chemistry format. 1 dozen Eg are 1000 g 1 dz, Bl are 2000 g 1 Eg 1000 g/dz 2 Bl 3 2000 g/dz Eg + Bl + Cp 1 dz Cp = 3000 g Cp 3000 g/dz g Eg 5 Bl 3 Cp

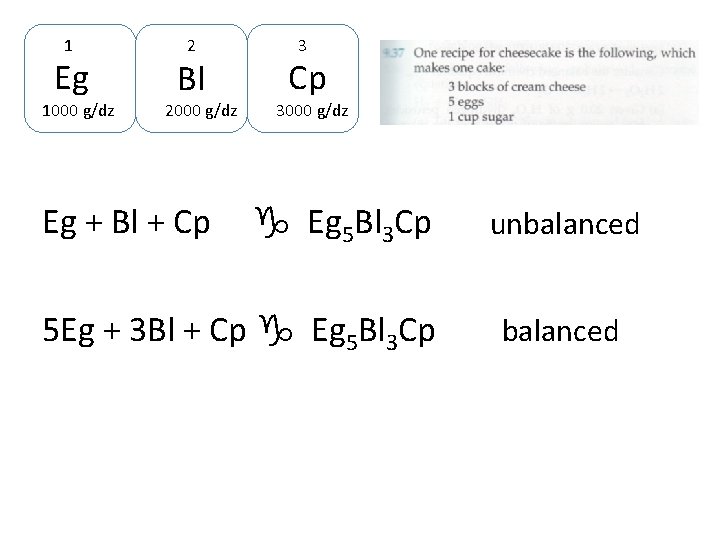
1 Eg 1000 g/dz 2 Bl 2000 g/dz Eg + Bl + Cp 3000 g/dz g Eg 5 Bl 3 Cp unbalanced

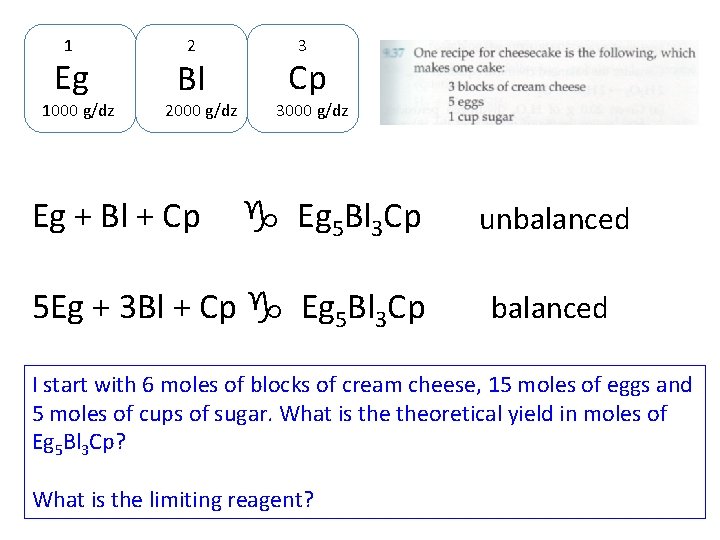
1 Eg 1000 g/dz 2 Bl 2000 g/dz Eg + Bl + Cp 3000 g/dz g Eg 5 Bl 3 Cp 5 Eg + 3 Bl + Cp g Eg 5 Bl 3 Cp unbalanced

1 Eg 1000 g/dz 2 Bl 2000 g/dz Eg + Bl + Cp 3000 g/dz g Eg 5 Bl 3 Cp 5 Eg + 3 Bl + Cp g Eg 5 Bl 3 Cp unbalanced I start with 6 moles of blocks of cream cheese, 15 moles of eggs and 5 moles of cups of sugar. What is theoretical yield in moles of Eg 5 Bl 3 Cp? What is the limiting reagent?

Compare I start with 6 blocks of cream cheese, 15 eggs and 5 cups of sugar. What is the maximum number of dozens of cheesecakes which I can make and which of the ingredients do I use up first? with 5 Eg + 3 Bl + Cp g Eg 5 Bl 3 Cp I start with 6 moles of blocks of cream cheese, 15 moles of eggs and 5 moles of cups of sugar. What is theoretical yield in moles of Eg 5 Bl 3 Cp? What is the limiting reagent?
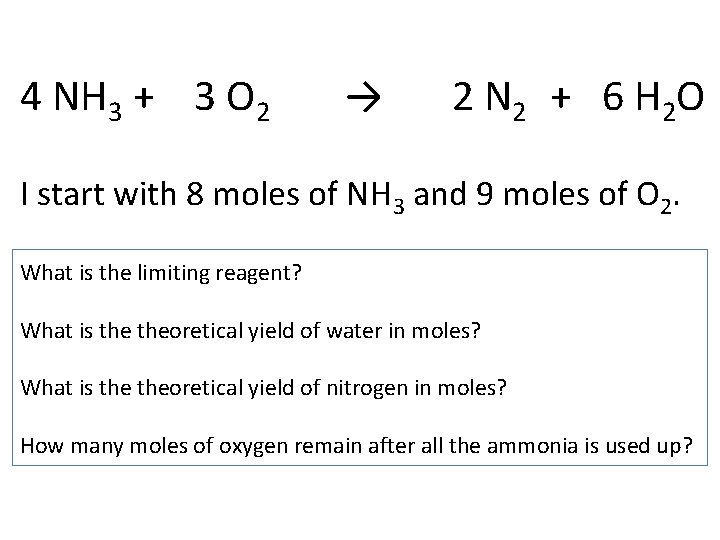
4 NH 3 + 3 O 2 → 2 N 2 + 6 H 2 O I start with 8 moles of NH 3 and 9 moles of O 2. What is the limiting reagent? What is theoretical yield of water in moles? What is theoretical yield of nitrogen in moles? How many moles of oxygen remain after all the ammonia is used up?
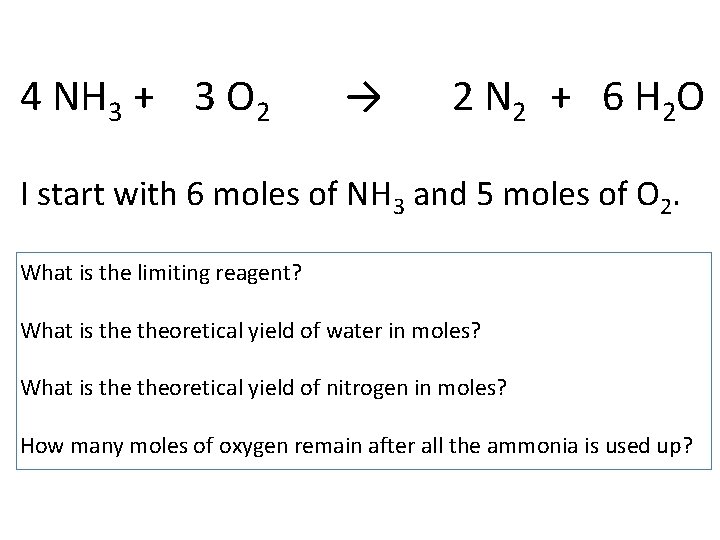
4 NH 3 + 3 O 2 → 2 N 2 + 6 H 2 O I start with 6 moles of NH 3 and 5 moles of O 2. What is the limiting reagent? What is theoretical yield of water in moles? What is theoretical yield of nitrogen in moles? How many moles of oxygen remain after all the ammonia is used up?

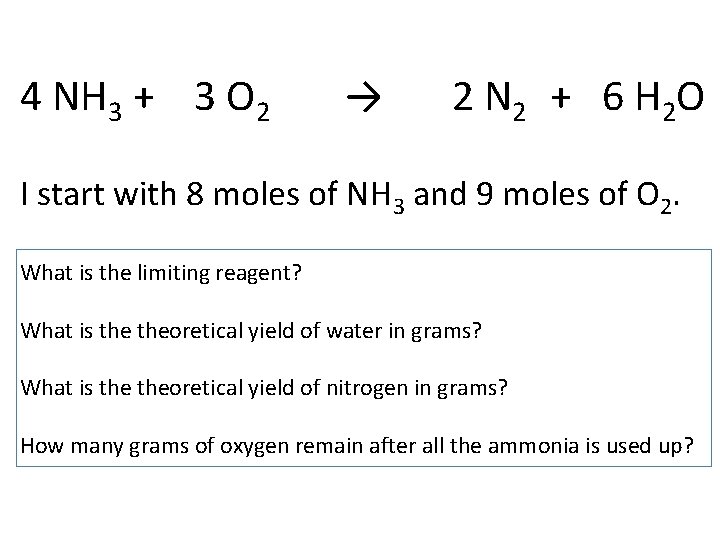
4 NH 3 + 3 O 2 → 2 N 2 + 6 H 2 O I start with 8 moles of NH 3 and 9 moles of O 2. What is the limiting reagent? What is theoretical yield of water in grams? What is theoretical yield of nitrogen in grams? How many grams of oxygen remain after all the ammonia is used up?

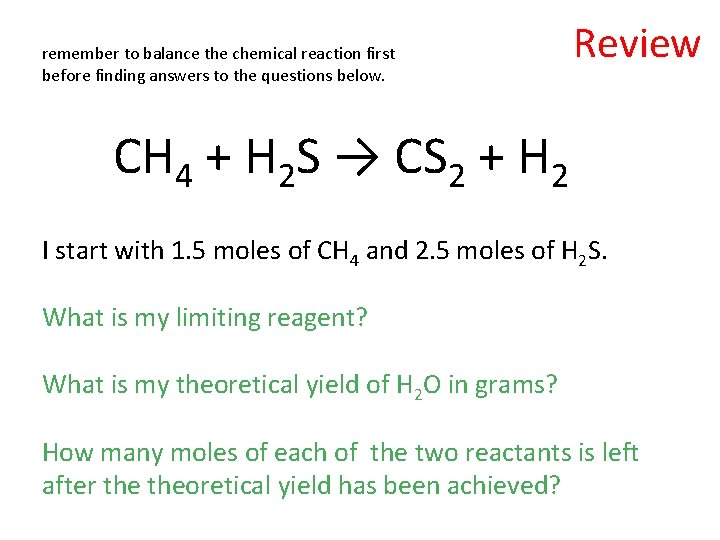
remember to balance the chemical reaction first before finding answers to the questions below. Review CH 4 + H 2 S → CS 2 + H 2 I start with 1. 5 moles of CH 4 and 2. 5 moles of H 2 S. What is my limiting reagent? What is my theoretical yield of H 2 O in grams? How many moles of each of the two reactants is left after theoretical yield has been achieved?

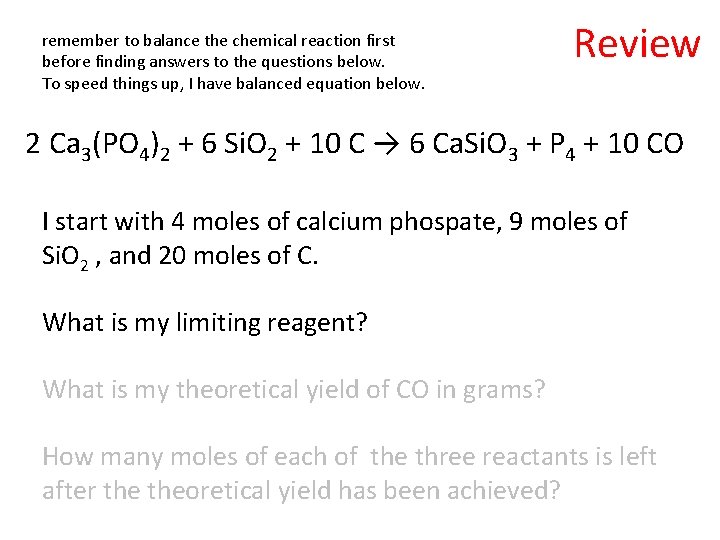
remember to balance the chemical reaction first before finding answers to the questions below. To speed things up, I have balanced equation below. Review 2 Ca 3(PO 4)2 + 6 Si. O 2 + 10 C → 6 Ca. Si. O 3 + P 4 + 10 CO I start with 4 moles of calcium phospate, 9 moles of Si. O 2 , and 20 moles of C. What is my limiting reagent? What is my theoretical yield of CO in grams? How many moles of each of the three reactants is left after theoretical yield has been achieved?

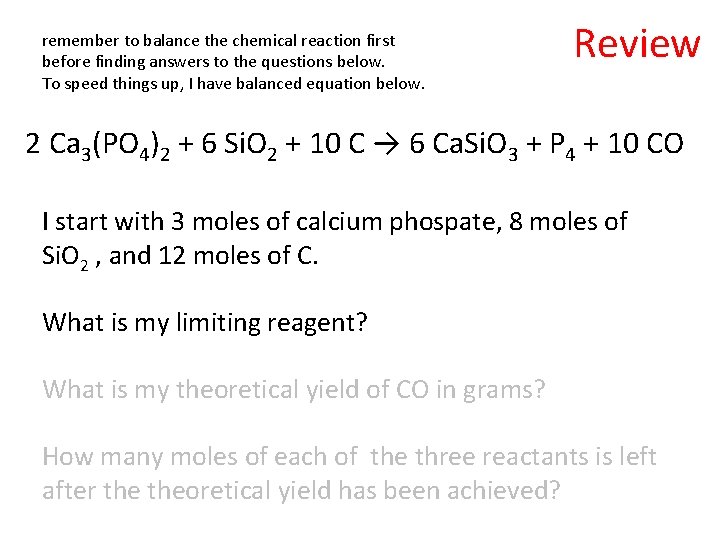
remember to balance the chemical reaction first before finding answers to the questions below. To speed things up, I have balanced equation below. Review 2 Ca 3(PO 4)2 + 6 Si. O 2 + 10 C → 6 Ca. Si. O 3 + P 4 + 10 CO I start with 3 moles of calcium phospate, 8 moles of Si. O 2 , and 12 moles of C. What is my limiting reagent? What is my theoretical yield of CO in grams? How many moles of each of the three reactants is left after theoretical yield has been achieved?

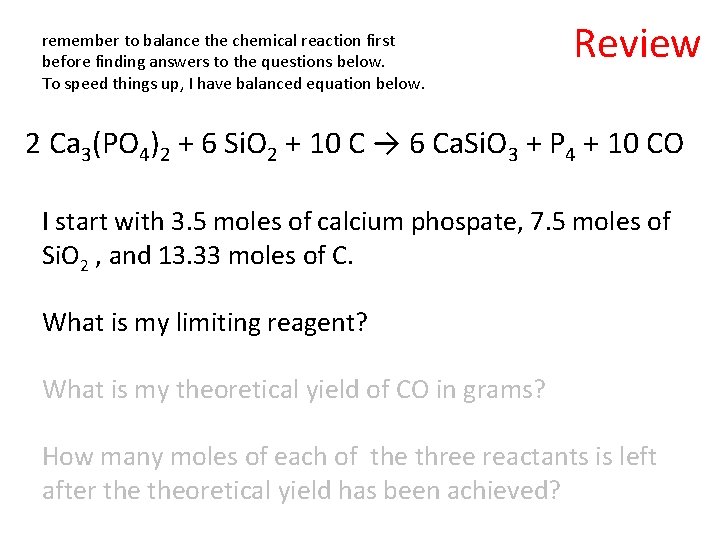
remember to balance the chemical reaction first before finding answers to the questions below. To speed things up, I have balanced equation below. Review 2 Ca 3(PO 4)2 + 6 Si. O 2 + 10 C → 6 Ca. Si. O 3 + P 4 + 10 CO I start with 3. 5 moles of calcium phospate, 7. 5 moles of Si. O 2 , and 13. 33 moles of C. What is my limiting reagent? What is my theoretical yield of CO in grams? How many moles of each of the three reactants is left after theoretical yield has been achieved?

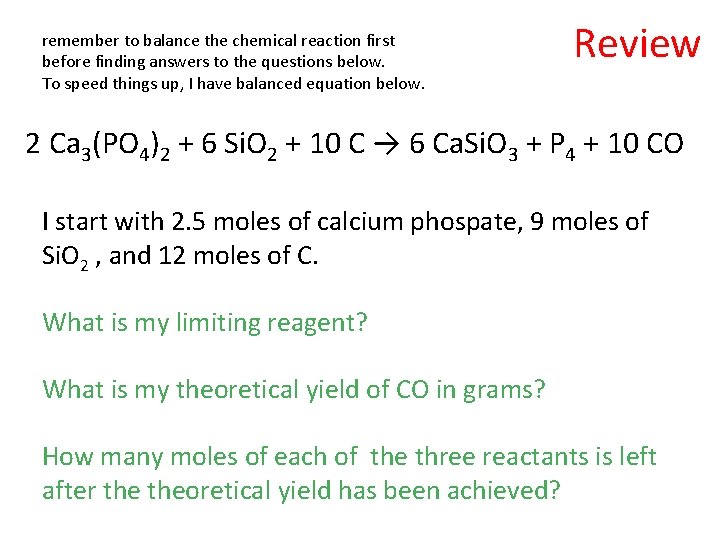
remember to balance the chemical reaction first before finding answers to the questions below. To speed things up, I have balanced equation below. Review 2 Ca 3(PO 4)2 + 6 Si. O 2 + 10 C → 6 Ca. Si. O 3 + P 4 + 10 CO I start with 2. 5 moles of calcium phospate, 9 moles of Si. O 2 , and 12 moles of C. What is my limiting reagent? What is my theoretical yield of CO in grams? How many moles of each of the three reactants is left after theoretical yield has been achieved?