QUALITY ASSURANCE Quality Assurance of Reagents Supplies And











- Slides: 18

QUALITY ASSURANCE Quality Assurance of Reagents, Supplies, And Laboratory Water

• In order to produce high quality work, a laboratory requires a constant supply of good quality reagents, supplies and water • A deficiency of any of these can cause the most efficient laboratory either to come to a standstill or to provide substandard service 2

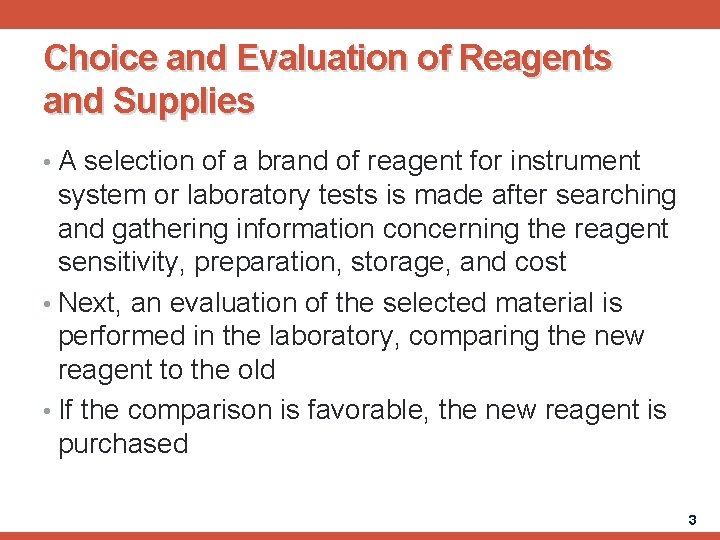
Choice and Evaluation of Reagents and Supplies • A selection of a brand of reagent for instrument system or laboratory tests is made after searching and gathering information concerning the reagent sensitivity, preparation, storage, and cost • Next, an evaluation of the selected material is performed in the laboratory, comparing the new reagent to the old • If the comparison is favorable, the new reagent is purchased 3

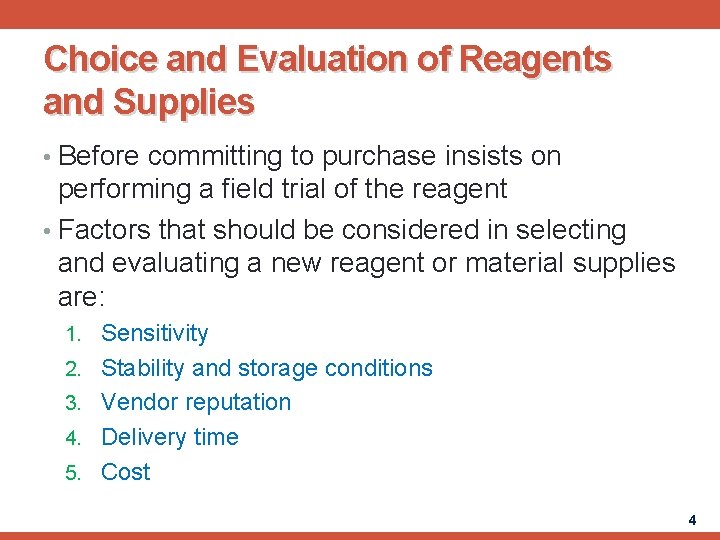
Choice and Evaluation of Reagents and Supplies • Before committing to purchase insists on performing a field trial of the reagent • Factors that should be considered in selecting and evaluating a new reagent or material supplies are: 1. Sensitivity 2. Stability and storage conditions 3. Vendor reputation 4. Delivery time 5. Cost 4

Laboratory Inventory Management • Effective inventory control involves setting up a system that has the following goals: • Simplify and reduce paperwork • Improve communication between the laboratory and the other hospital departments involved in purchasing, stocking, and paying for supplies • Manage inventory so that Shortages and overstocking should be avoided • Teaching laboratory employees better budgeting and materials management techniques 5

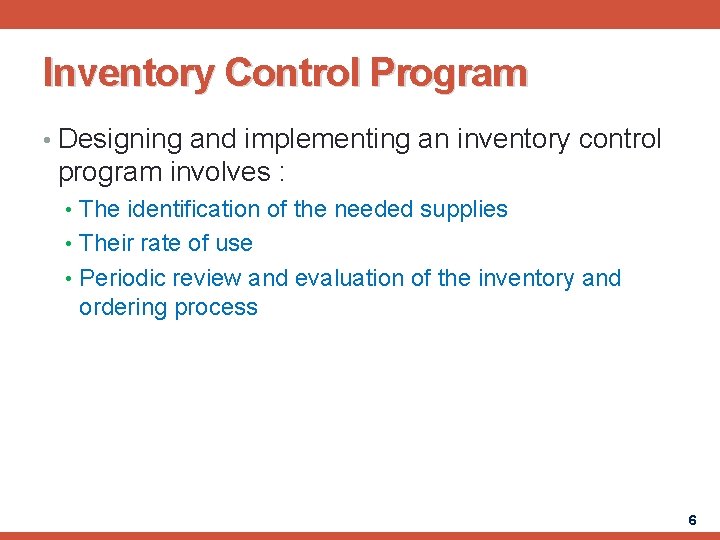
Inventory Control Program • Designing and implementing an inventory control program involves : • The identification of the needed supplies • Their rate of use • Periodic review and evaluation of the inventory and ordering process 6

Inventory Control Program • Conduct a survey to list all of supplies that the laboratory uses, the list should include: • The name of the item • A brief description • Approximate usage per month • Current vendor • Order unit amount • Current unit packing, that is per box, carton, or bag • Order or catalogue number • Priority of need • Assigning an item its relative importance • High priority: if needed constantly or cannot be done without • Medium priority: if needed occasionally • Low priority: if needed rarely 7

Inventory Control Program • The next step is to determine the order point, order quantity and lead time for the item • Order point: the sum of the minimum inventory plus the emergency supply • The level of inventory at which an order is generated • Lead Time: is the length of time between initiating an order and receiving it in the laboratory 8

Inventory Control Program • Controlling inventory involves the counting, storage and movement of supplies within the laboratory • A written record system of inventory levels and checks should be devised • Three types of record systems are suggested: 1. The periodic count • Strict inventory control is not required • Count of materials weekly or every two weeks, when count reaches the order point, an order is generated • Good for small to midsize Lab. 9

Inventory Control Program 2. The perpetual inventory record • Strict inventory control is required • Inventory is closed to lab. Personnel, one or two persons manage it • Good for larger lab. 3. The specialized inventory record • Used for slow-moving, infrequently ordered parts such as instrument spare parts • Parts are not ordered until inventory is used • Once the inventory system has been set up, it should be reviewed annually for updating 10

Reagents Prepared in the Lab. • Reagents, standards and controls prepared in the laboratory from stock chemicals should be: • Prepared using class A volumetric glassware and properly calibrated balances • To eliminate variation (batch to batch), preparation should be limited to one or two persons • Label each reagent, standard, and control with the following: • The name of the material • The procedure for which the material is to be used • Date of preparation • Date of expiration • Initials of the person who prepared it 11

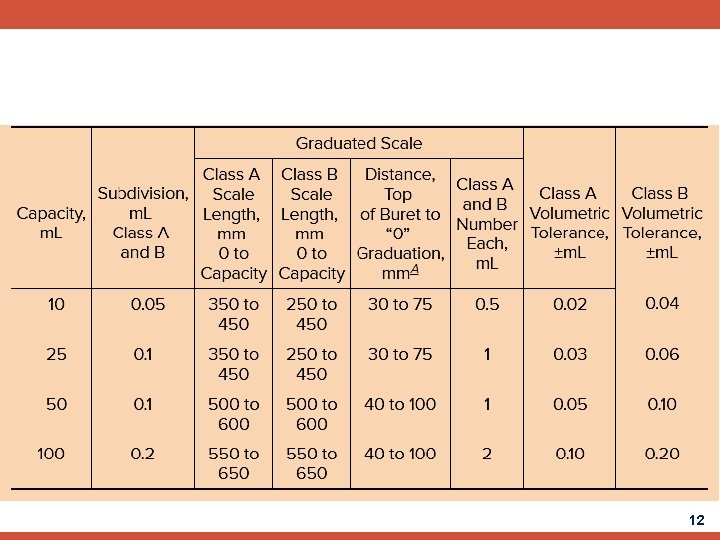
12

Laboratory Water • Reagent - grade water must be properly purified and periodically inspected for: • Electrical resistivity • Resistivity (R) of water is the measurement of electrical resistance and is the inverse of electrical conductivity (C) • R and C are directly related to the number of inorganic ions and conduction particles in the water • The greater the ionic concentration, the greater the electrical conductivity and the less the electrical resistance • Measurements are made using a resistivity or conductivity meter • Soluble silica concentration • measured by a chemical reaction between silicate and molybdate ions to form a blue complex 13

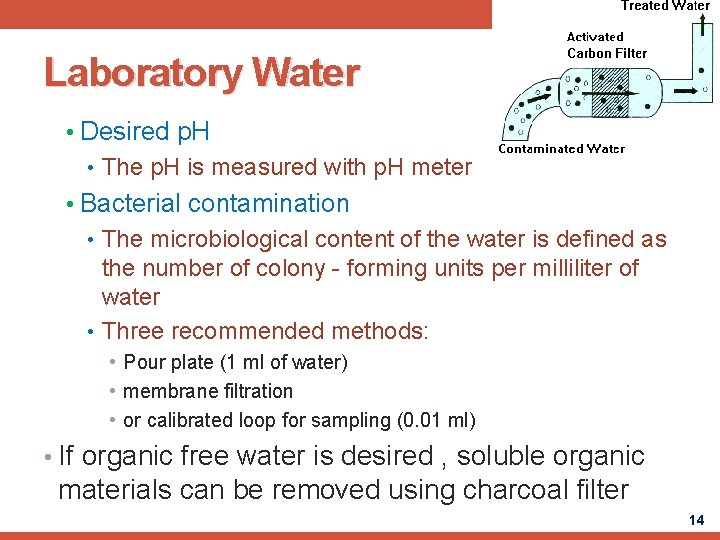
Laboratory Water • Desired p. H • The p. H is measured with p. H meter • Bacterial contamination • The microbiological content of the water is defined as the number of colony - forming units per milliliter of water • Three recommended methods: • Pour plate (1 ml of water) • membrane filtration • or calibrated loop for sampling (0. 01 ml) • If organic free water is desired , soluble organic materials can be removed using charcoal filter 14

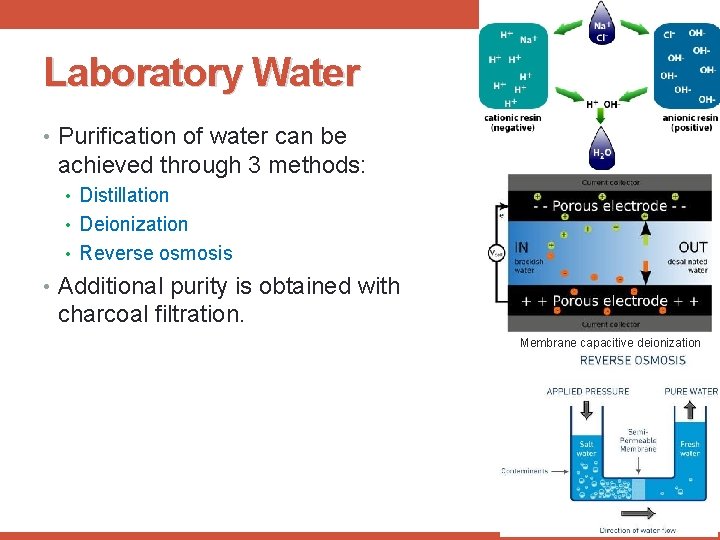
Laboratory Water • Purification of water can be achieved through 3 methods: • Distillation • Deionization • Reverse osmosis • Additional purity is obtained with charcoal filtration. Membrane capacitive deionization 15

Water Grades According to Purity • Type I water: • The highest level of purity, • used for: • tissue and cell culture methods • special and critical analytical chemical analysis • and in preparation of standard solutions • Type II water: • Used for most routine quantitative clinical laboratory methods • It should be stored for short periods of time before use, to prevent change in resistivity and bacterial growth 16

Water Grades According to Purity • Type III water: • The least pure • Suitable for most qualitative procedures including: • Urine analysis • parsitology • and histology • Suitable for glassware washing • Stored in containers that protect it form contamination 17

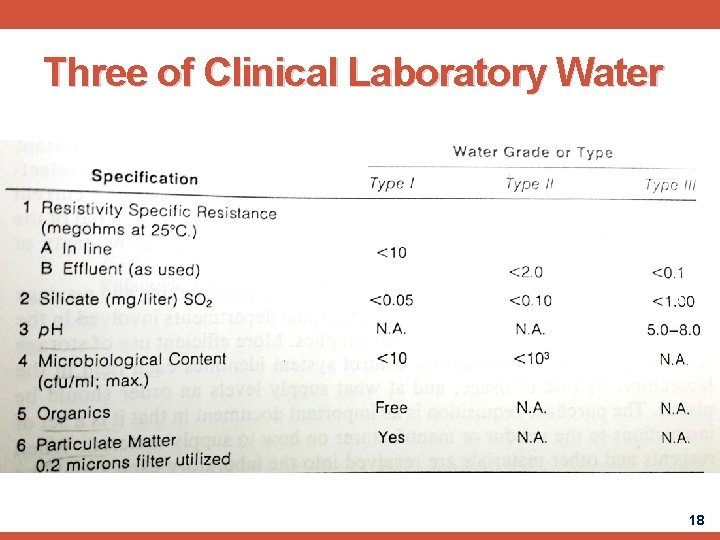
Three of Clinical Laboratory Water 18
 Quality control of blood components
Quality control of blood components Quality control of reagents
Quality control of reagents Quality control and quality assurance
Quality control and quality assurance Quality control basics
Quality control basics Project quality management pmp
Project quality management pmp Pmbok quality assurance vs quality control
Pmbok quality assurance vs quality control Total quality management seminar
Total quality management seminar Quality improvement vs quality assurance
Quality improvement vs quality assurance Urease test salmonella
Urease test salmonella How to calculate limiting reagent
How to calculate limiting reagent Limiting reactant shortcut
Limiting reactant shortcut Kinetics flotation reagents
Kinetics flotation reagents Benzidine test used for
Benzidine test used for Protein denaturation
Protein denaturation Oxymercuration of alkynes
Oxymercuration of alkynes Advia 120
Advia 120 The polar group
The polar group Quality assurance theory
Quality assurance theory Quality revolution
Quality revolution