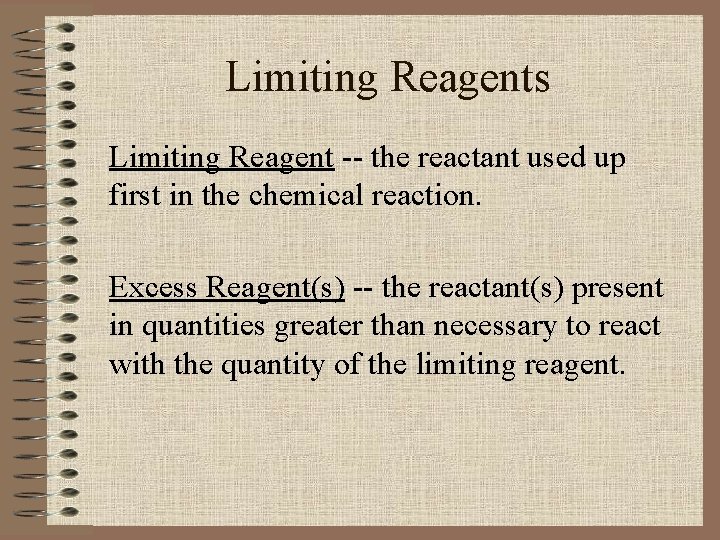
Limiting Reagents Limiting Reagent the reactant used up






- Slides: 17

Limiting Reagents Limiting Reagent -- the reactant used up first in the chemical reaction. Excess Reagent(s) -- the reactant(s) present in quantities greater than necessary to react with the quantity of the limiting reagent.

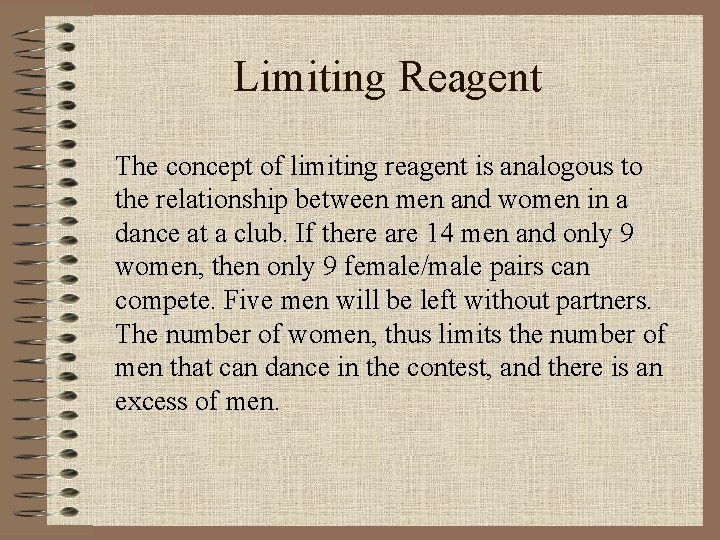
Limiting Reagent The concept of limiting reagent is analogous to the relationship between men and women in a dance at a club. If there are 14 men and only 9 women, then only 9 female/male pairs can compete. Five men will be left without partners. The number of women, thus limits the number of men that can dance in the contest, and there is an excess of men.

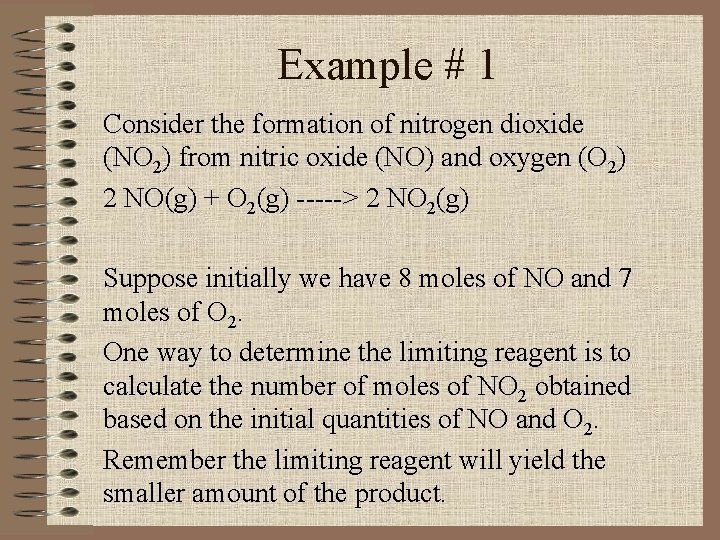
Example # 1 Consider the formation of nitrogen dioxide (NO 2) from nitric oxide (NO) and oxygen (O 2) 2 NO(g) + O 2(g) -----> 2 NO 2(g) Suppose initially we have 8 moles of NO and 7 moles of O 2. One way to determine the limiting reagent is to calculate the number of moles of NO 2 obtained based on the initial quantities of NO and O 2. Remember the limiting reagent will yield the smaller amount of the product.

Calculate the number of moles of product obtained from each reactant. The one that yields the smallest amount of product is the limiting reagent.

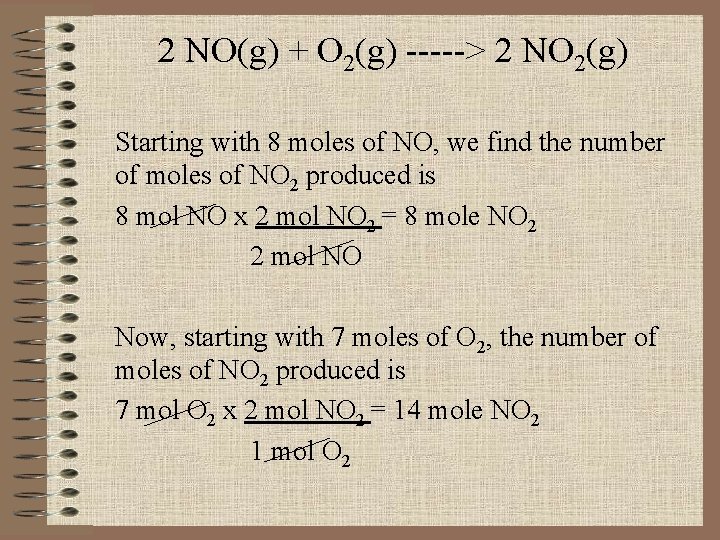
2 NO(g) + O 2(g) -----> 2 NO 2(g) Starting with 8 moles of NO, we find the number of moles of NO 2 produced is 8 mol NO x 2 mol NO 2 = 8 mole NO 2 2 mol NO Now, starting with 7 moles of O 2, the number of moles of NO 2 produced is 7 mol O 2 x 2 mol NO 2 = 14 mole NO 2 1 mol O 2

Because NO yields the smaller amount of NO 2, it must be the limiting reagent. Therefore, O 2 is the excess reagent.

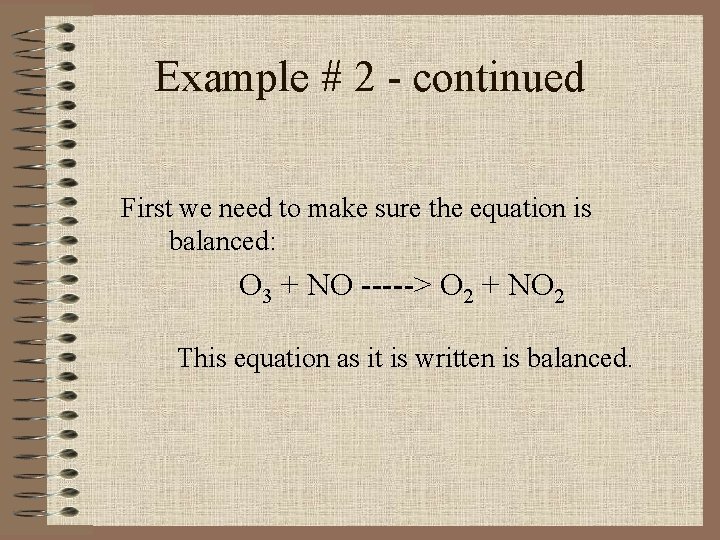
Example # 2 The depletion of ozone (O 3) in the stratosphere has been a matter of great concern among scientists in recent years. It is believed that ozone can react with nitric oxide (NO) that is discharged from the high-altitude jet plane, the SST. The reaction is O 3 + NO -----> O 2 + NO 2

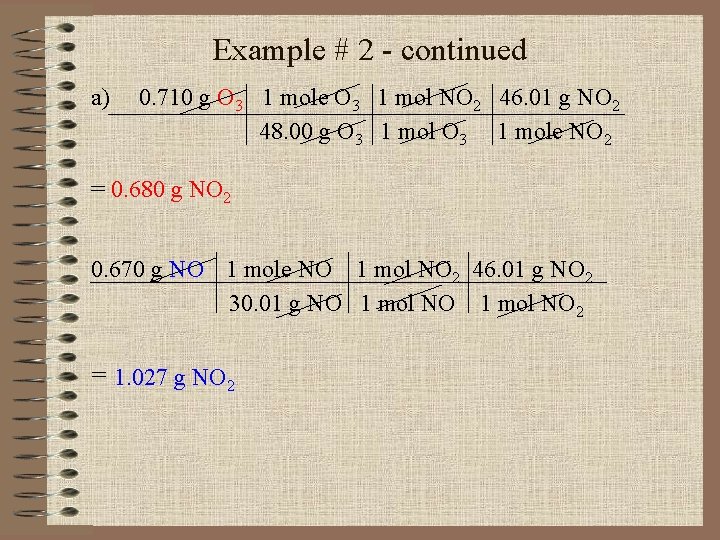
Example # 2 - continued If 0. 710 g of O 3 reacts with 0. 670 g of NO, a) how many grams of NO 2 will be produced? b) Which compound is the limiting reagent? c) Calculate the number of grams of the excess reagent remaining at the end of the reaction?

Example # 2 - continued First we need to make sure the equation is balanced: O 3 + NO -----> O 2 + NO 2 This equation as it is written is balanced.

Example # 2 - continued a) 0. 710 g O 3 1 mole O 3 1 mol NO 2 46. 01 g NO 2 48. 00 g O 3 1 mole NO 2 = 0. 680 g NO 2 0. 670 g NO 1 mole NO 1 mol NO 2 46. 01 g NO 2 30. 01 g NO 1 mol NO 2 = 1. 027 g NO 2

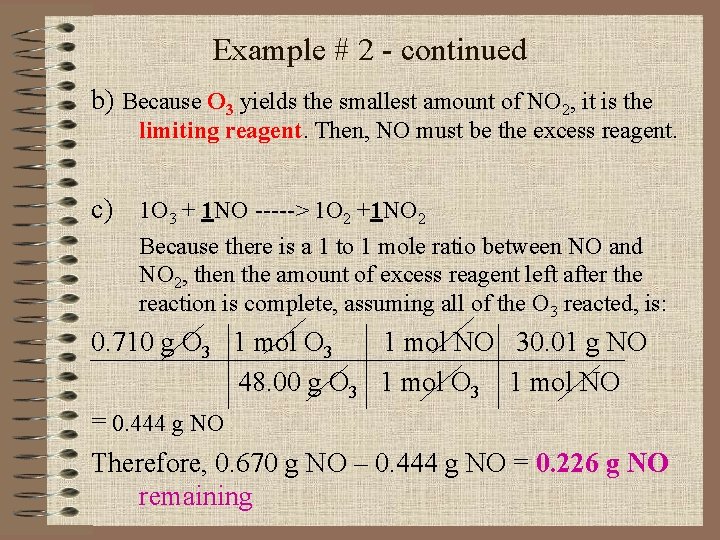
Example # 2 - continued b) Because O 3 yields the smallest amount of NO 2, it is the limiting reagent. Then, NO must be the excess reagent. c) 1 O 3 + 1 NO -----> 1 O 2 +1 NO 2 Because there is a 1 to 1 mole ratio between NO and NO 2, then the amount of excess reagent left after the reaction is complete, assuming all of the O 3 reacted, is: 0. 710 g O 3 1 mol NO 30. 01 g NO 48. 00 g O 3 1 mol NO = 0. 444 g NO Therefore, 0. 670 g NO – 0. 444 g NO = 0. 226 g NO remaining

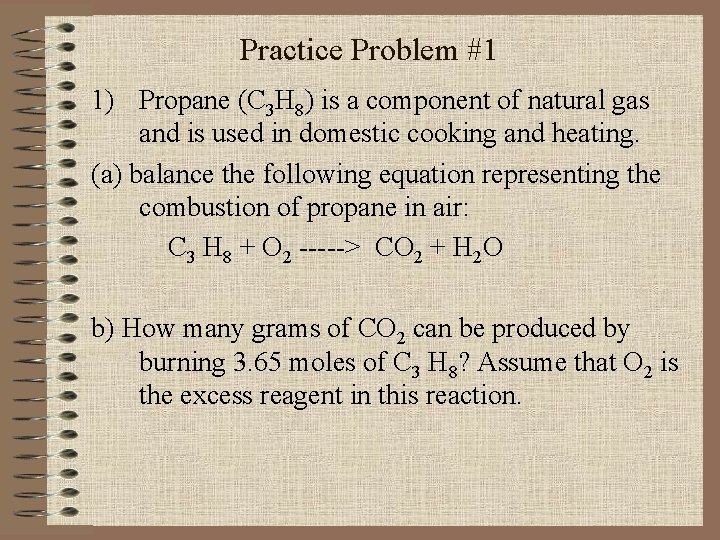
Practice Problem #1 1) Propane (C 3 H 8) is a component of natural gas and is used in domestic cooking and heating. (a) balance the following equation representing the combustion of propane in air: C 3 H 8 + O 2 -----> CO 2 + H 2 O b) How many grams of CO 2 can be produced by burning 3. 65 moles of C 3 H 8? Assume that O 2 is the excess reagent in this reaction.

Practice Problem #2 2) Consider the reaction Mn. O 2 + 4 HCl ----> Mn. Cl 2 + 2 H 2 O If 0. 86 mole of Mn. O 2 and 48. 2 g of HCl react, a) Which reagent will be used up first? Show your work. b) How many grams of Cl 2 will be produced?

Practice Problem #3 3) In the production of disulfur dichloride, molten sulfur reacts with chlorine gas according to the equation below: S 8 (l) + 4 Cl 2(g) ----> 4 SCl 2(l) If 200. 0 g of S react with 100. 0 g of Cl 2, a) what mass of disulfur dichloride is produced? b) What reactant is the limiting reagent? Show your work. c) What reactant is the excess reagent? Show your work. d) How much of the excess reagent is left in grams?

Practice Problem #4 4)The reaction between solid white phosphorus and oxygen produces solid tetraphosphorus decoxide (P 4 O 10). a) Write the equation and balance it. Hint use the info. below to determine the reagents. b) Determine the mass of P 4 O 10 formed if 25. 0 g of P 4 and 50. 0 g of O 2 are combined. c) What is the limiting reagent? Show your work. d) How much of the excess reactant remains after the reaction stops?

Practice Problem #5 5) The reaction between solid sodium and iron(III)oxide is one is a series of reactions that inflates an automobile airbag. 6 Na(s) + Fe 2 O 3(s) -----> 3 Na 2 O(s) + 2 Fe(s) If 100. 0 g Na and 100. 0 g Fe 2 O 3 are used in this reaction, determine a) The limiting reagent. b) The reactant in excess. c) The mass of solid iron produced. d) The mass of excess reactant that remains after the reaction is complete.

Practice Problem #6 6) Photosynthesis reactions in green plants use carbon dioxide and water to produce glucose (C 6 H 12 O 6) and oxygen. a) Write the balanced equation. b) If a plant had 88. 0 g of carbon dioxide and 64. 0 g water available for photosynthesis, determine i. The limiting reactant ii. The excess reactant and the mass in excess iii. The mass of glucose produced