Thermodynamics of Surfaces and Interfaces What is thermodynamics

























- Slides: 46

Thermodynamics of Surfaces and Interfaces

What is thermodynamics dealing with? • Thermodynamics is the branch of science that id concerned with the principles of energy transformation in macroscopic system. • Macroscopic properties of matter arise from the behavior of a very large number of molecules. • Thermodynamics is based upon experiments and observation , summarized and generalized in the laws of thermodynamics. • These laws are not derivable from any other principles: they are in fact improvable and therefore can be regarded as assumptions only.

Some Definitions • • Intensive variables Extensive variables System, isolated, open, closed Surroundings Boundary Equilibrium Process Thermodynamic state

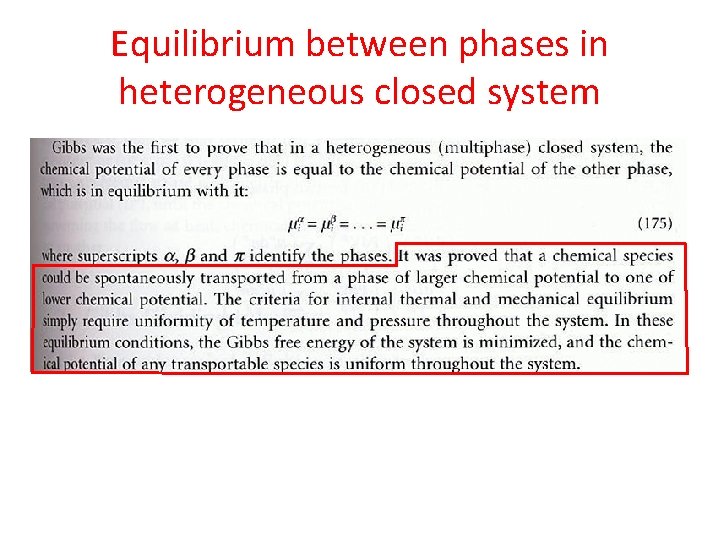
Equilibrium between phases in heterogeneous closed system • What does equilibrium mean? – A system is in eq if no further spontaneous changes takes place and if the same state can be reached by other directions. • A phase? • A phase equilibrium is defined when the same species are present in different phases. • A heterogeneous closed systems is composed of two or more phases, with each phase is considered as an open system within an overall closed system.

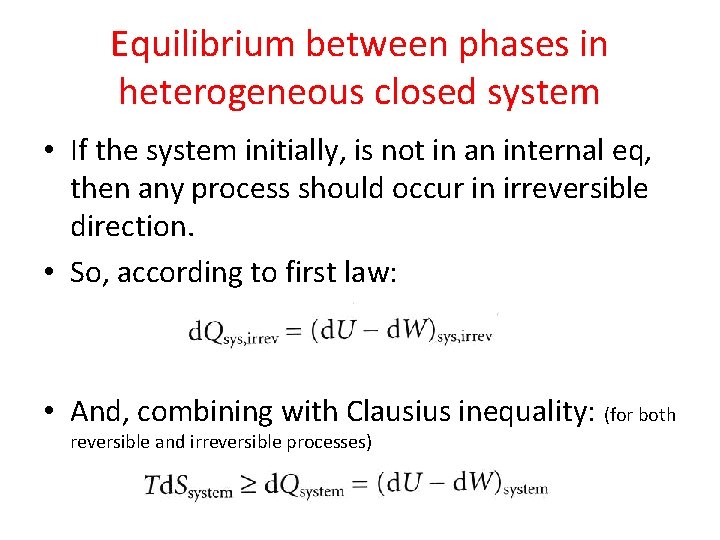
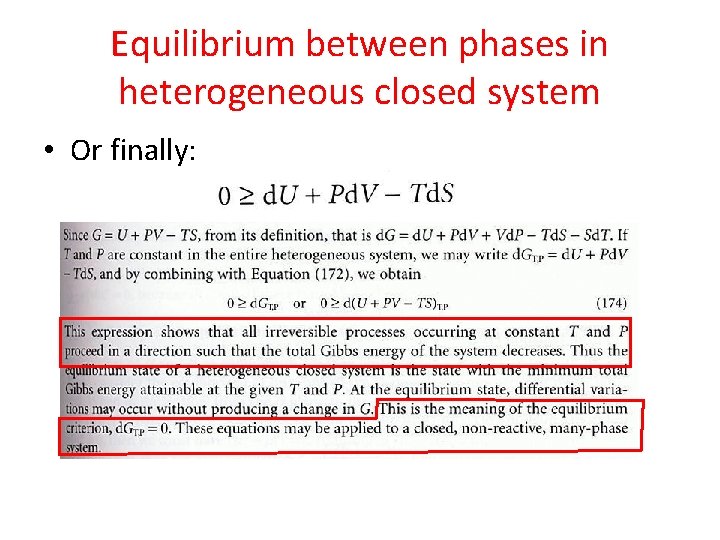
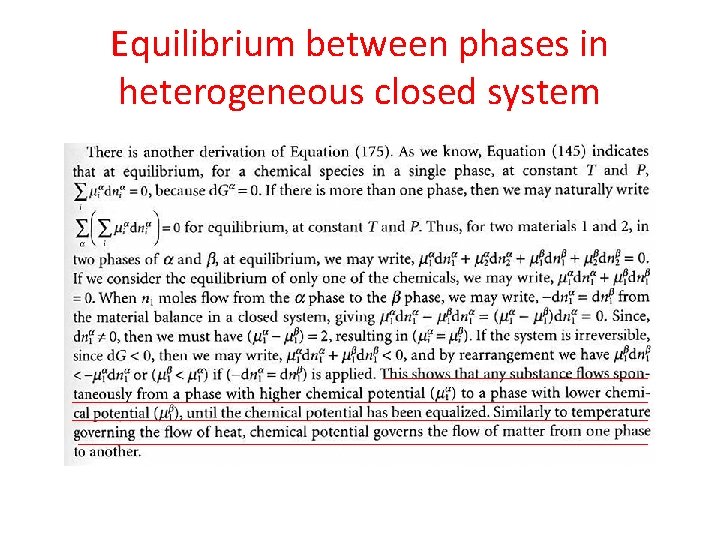
Equilibrium between phases in heterogeneous closed system • If the system initially, is not in an internal eq, then any process should occur in irreversible direction. • So, according to first law: • And, combining with Clausius inequality: (for both reversible and irreversible processes)

Equilibrium between phases in heterogeneous closed system • Or finally:

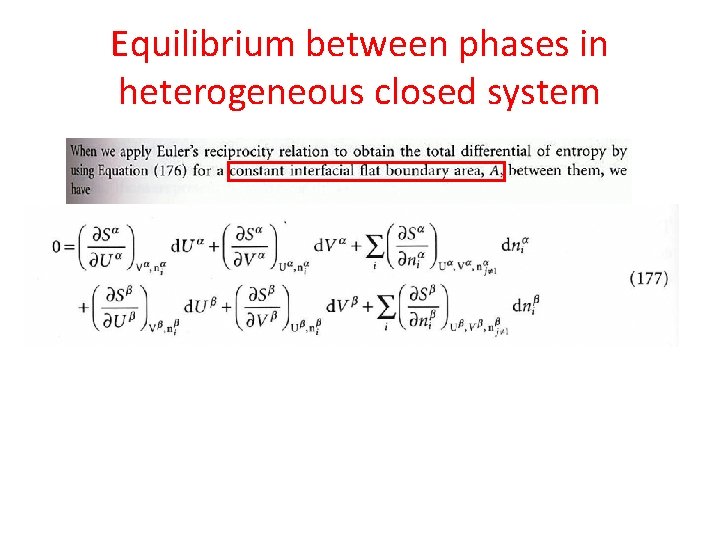
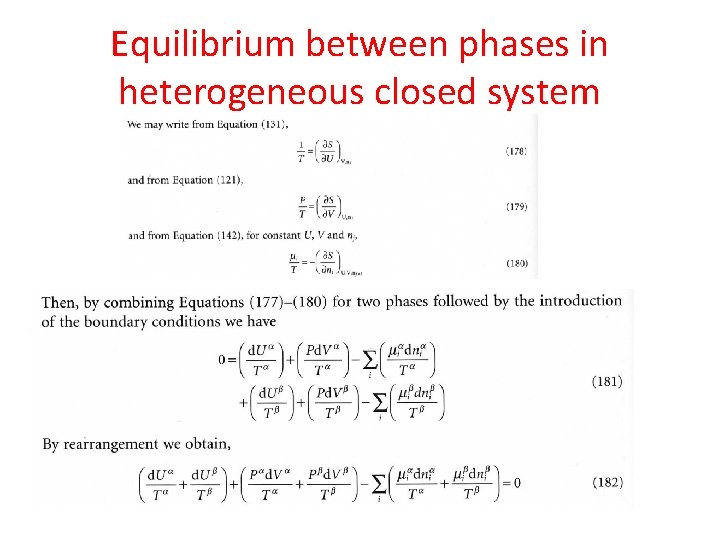
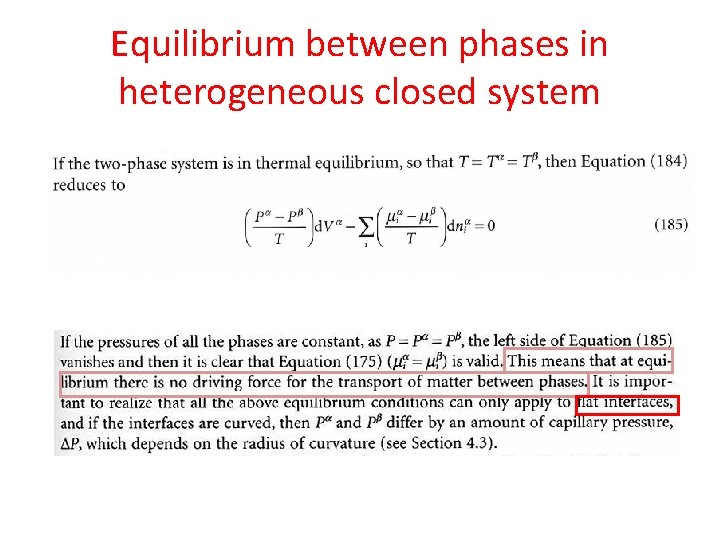
Equilibrium between phases in heterogeneous closed system

Equilibrium between phases in heterogeneous closed system

Equilibrium between phases in heterogeneous closed system

Equilibrium between phases in heterogeneous closed system

Equilibrium between phases in heterogeneous closed system

Equilibrium between phases in heterogeneous closed system













Wettability and Contact Angle Reference:

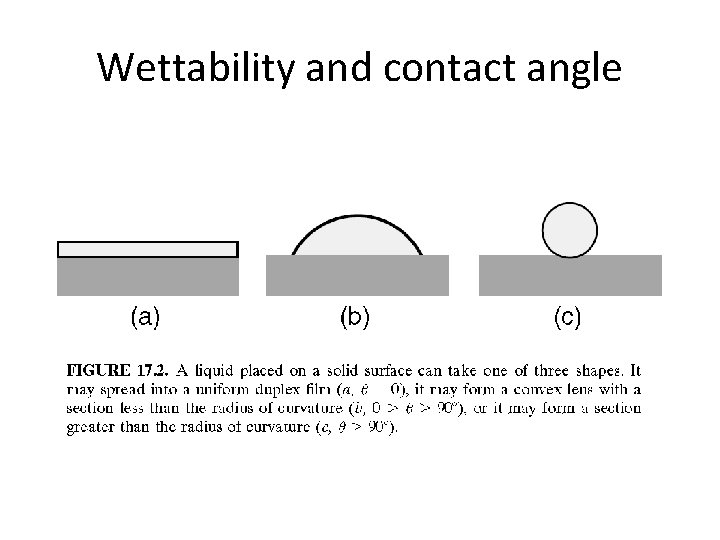
Wettability and contact angle

• In the case of a liquid that forms a uniform film (i. e. , where � = 0), the solid is said to be completely wetted by the liquid, or that the liquid wets the solid. • Where a nonzero angle is formed, there exists some controversy as to how to describe the system. If a finite contact angle is formed (� > 0), some investigators describe the system as being partially wetted.

• Others prefer to make a distinction based on the size of the contact angle. For example, a given worker may define as ‘‘wetting’’ any liquid that produces a contact angle of 30 or less on a solid. Between 30 and 89 the system would be ‘‘partially wetting, ’’ and 90 and above nonwetting. • Alternatively, any system with 0<� < 180 would be partially wetting, and only for 180 would the nonwetting term be applied.

• While the contact angle of a liquid on a solid may be considered a characteristic of the system, that will be true only if the angle is measured under specified conditions of equilibrium, time, temperature, component purity, and other parameters. • The great utility of contact angle measurements stems from their interpretation based on equilibrium thermodynamic considerations. As a result, most studies are conducted on essentially static systems in which the liquid drop has (presumably) been allowed to come to its final equilibrium value under controlled conditions.

• As an application, contact angles, for example, can be extremely useful as aspot test of the cleanliness of sensitive surfaces such as glass or silicon wafers for microelectronics fabrications. • Both surfa es are ‘‘high energy’’ and are completely wetted by pure water. • If the surface is contaminated by something such as an oil that interferes with the processing of the material (e. g. , the coating of a photoresist polymer), a drop of water will have a nonzero contact angle, and the contamination will be immediately apparent.

Contact angle hysteresis • For systems that have ‘‘true’’ nonzero contact angles, the situation may be further complicated by the existence of contact angle hysteresis. • Thus, the contact angle one observes may vary depending on whether the liquid is advancing across fresh surface (the advancing contact angle, A) or receding from an already wetted surface (the receding contact angle, R) (Fig. 17. 3).


• As an operational convenience, many, if not most, static contact angles measured and reported are in fact advancing angles. For a given system, it will be found that � ��� A� R. • In practice, very few systems exhibit a complete lack of hysteresis, so that the problem can be operational as well as philosophical. • One should keep in mind that when discussing contact angle data, one must always be aware of how the angle has been measured in order to interpret its significance properly.

Why hysteresis • In dynamic contact angle studies, additional complications arise because the movement of the wetting line is not always a steady, continuous process. • It is often observed that the movement is ‘‘jerky, ’’ with the drop or liquid front holding a position for a time and then jumping to a new configuration. • This phenomenon is often referred to as a ‘‘stick–slip process’’ and is not fully understood as yet. It has also been observed that in dynamic systems, the values of � A and � R will vary as a function of the velocity of wetting line movement, with � A increasing with velocity and � R decreasing.

Why hysteresis • When used with Young’s equation and other such relationships, the contact angle provides a relatively simple yet sensitive insight into the general chemical nature of a surface through such thermodynamic quantities as the work of adhesion. Unfortunately, as already mentioned, contact angles often exhibit hysteresis and cannot be defined unambiguously by experiment. • It is always important to know as much as possible about the cleanliness, topography, homogeneity, and other characteristics of a solid surface, as well as the purity and composition of the liquid employed, when attempting to interpret contact angle data.

Why hysteresis • Although the existence of contact angle hysteresis has been recognized for at least 100 years, the root of the ‘‘evil’’ has not always been understood. In addition to the physicochemical adsorption process already mentioned, which leads to differences in advancing and receding contact angles, it is recognized that several physical and kinetic factors also contribute to the overall problem.

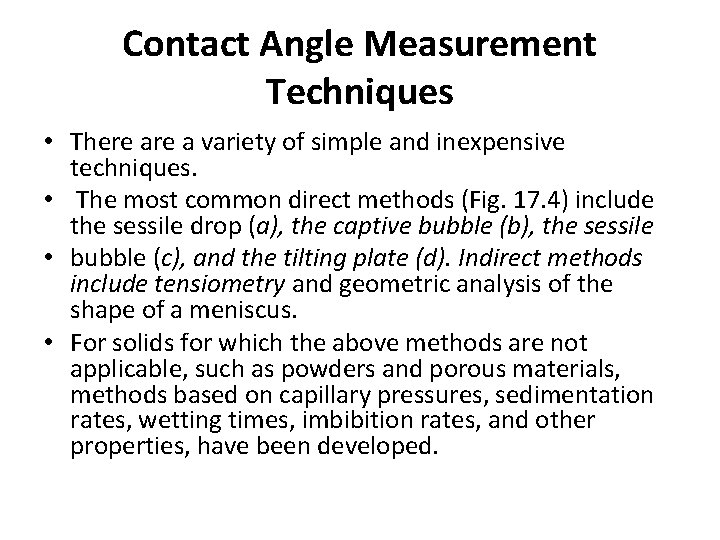
Contact Angle Measurement Techniques • There a variety of simple and inexpensive techniques. • The most common direct methods (Fig. 17. 4) include the sessile drop (a), the captive bubble (b), the sessile • bubble (c), and the tilting plate (d). Indirect methods include tensiometry and geometric analysis of the shape of a meniscus. • For solids for which the above methods are not applicable, such as powders and porous materials, methods based on capillary pressures, sedimentation rates, wetting times, imbibition rates, and other properties, have been developed.

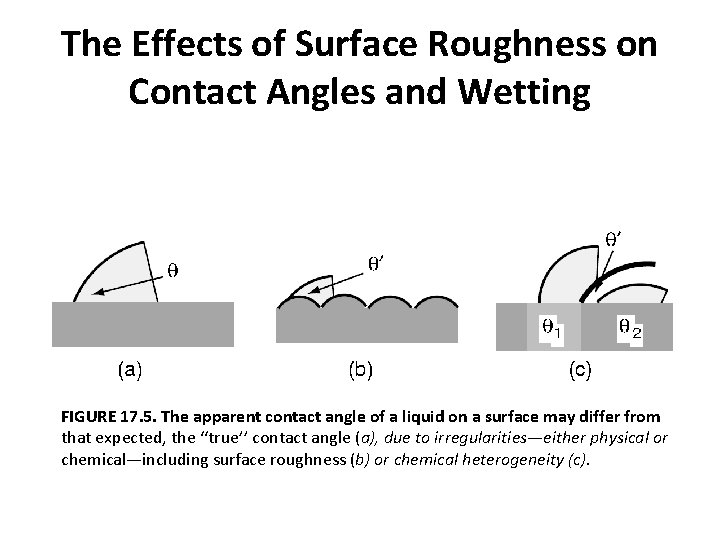
The Effects of Surface Roughness on Contact Angles and Wetting FIGURE 17. 5. The apparent contact angle of a liquid on a surface may differ from that expected, the ‘‘true’’ contact angle (a), due to irregularities—either physical or chemical—including surface roughness (b) or chemical heterogeneity (c).

The Effects of Surface Roughness on Contact Angles and Wetting • The theoretical discussion of contact angle and wetting to this point has assumed implicitly that the solid surface in question is a smooth, ideal plane. • In fact, of course, very few solid surfaces even begin to approach such a state. • The finest polished glass surface, for example, will usually have asperities of 5 nm or more. • Commonly encountered polished surfaces, will be much rougher • by factors of 10– 1000. • The earliest, and still most useful, quantitative attempt to correlate the observed contact angle of a liquid on a solid with the surface roughness is the Wenzel relationship which proposes a thermodynamic relationship such that

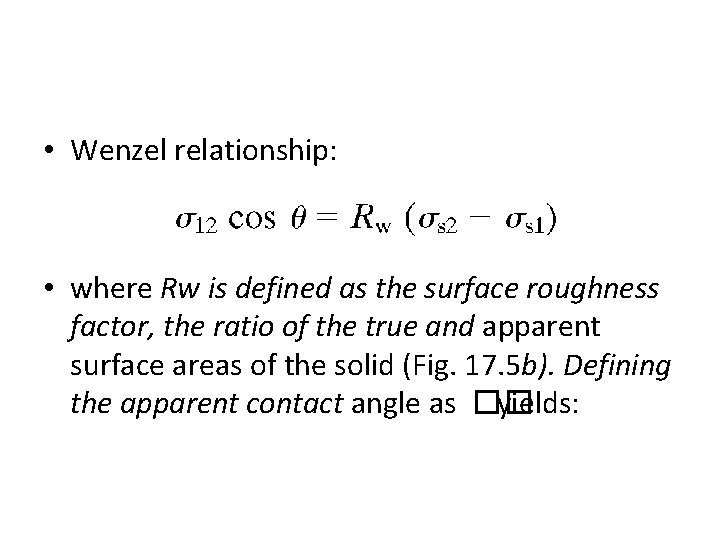
• Wenzel relationship: • where Rw is defined as the surface roughness factor, the ratio of the true and apparent surface areas of the solid (Fig. 17. 5 b). Defining the apparent contact angle as �� yields:

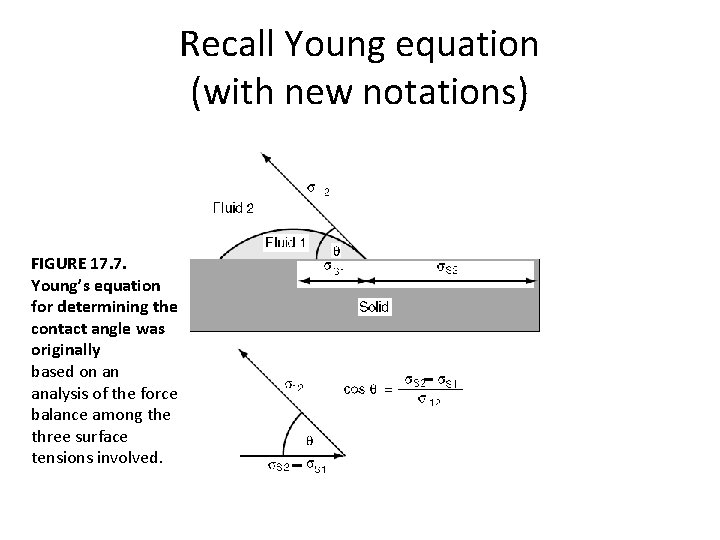
Recall Young equation (with new notations) FIGURE 17. 7. Young’s equation for determining the contact angle was originally based on an analysis of the force balance among the three surface tensions involved.

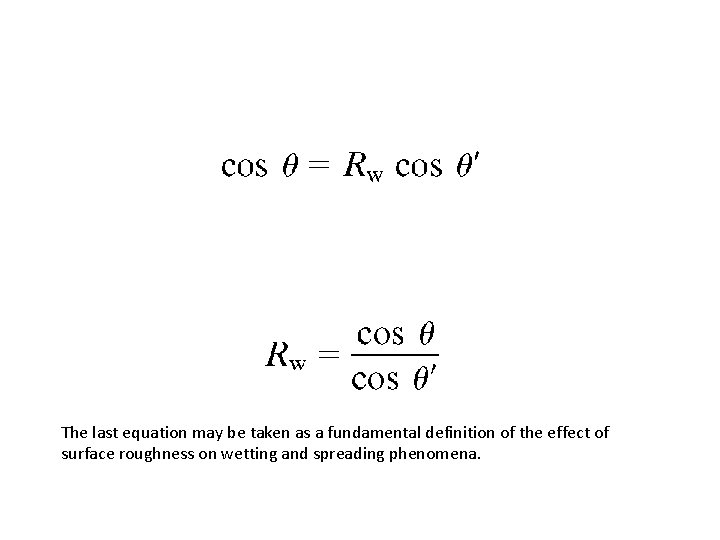
The last equation may be taken as a fundamental definition of the effect of surface roughness on wetting and spreading phenomena.

• As a final note on the effects of surface roughness, examination of the Equation, leads to a useful rule of thumb for some important applications of wetting and spreading phenomena; that is: • If the ‘‘true’’ contact angle of a liquid (an adhesive, say) is less than 90 on the smooth surface, the angle will be even smaller on a rough surface. • For a true contact angle 90, roughness will increase the apparent angle. Mathematically the situation can be described as:

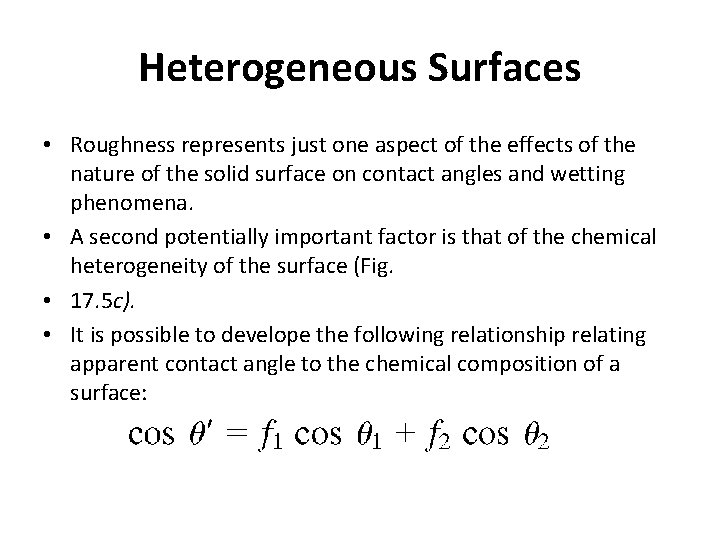
Heterogeneous Surfaces • Roughness represents just one aspect of the effects of the nature of the solid surface on contact angles and wetting phenomena. • A second potentially important factor is that of the chemical heterogeneity of the surface (Fig. • 17. 5 c). • It is possible to develope the following relationship relating apparent contact angle to the chemical composition of a surface:

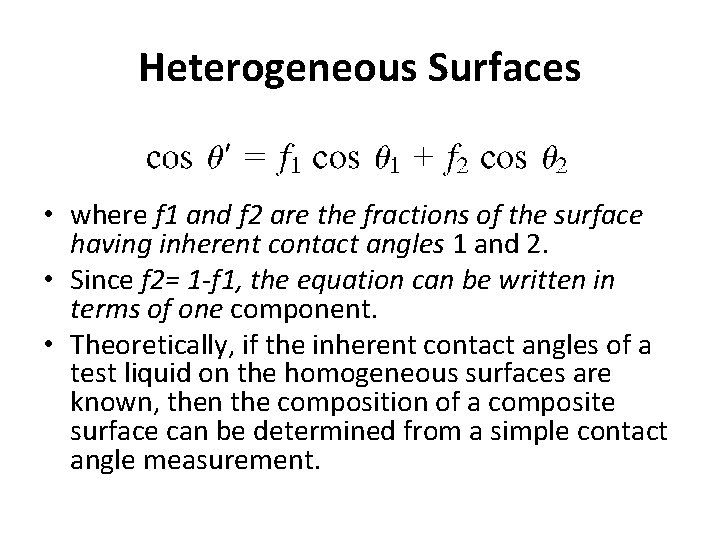
Heterogeneous Surfaces • where f 1 and f 2 are the fractions of the surface having inherent contact angles 1 and 2. • Since f 2= 1 -f 1, the equation can be written in terms of one component. • Theoretically, if the inherent contact angles of a test liquid on the homogeneous surfaces are known, then the composition of a composite surface can be determined from a simple contact angle measurement.

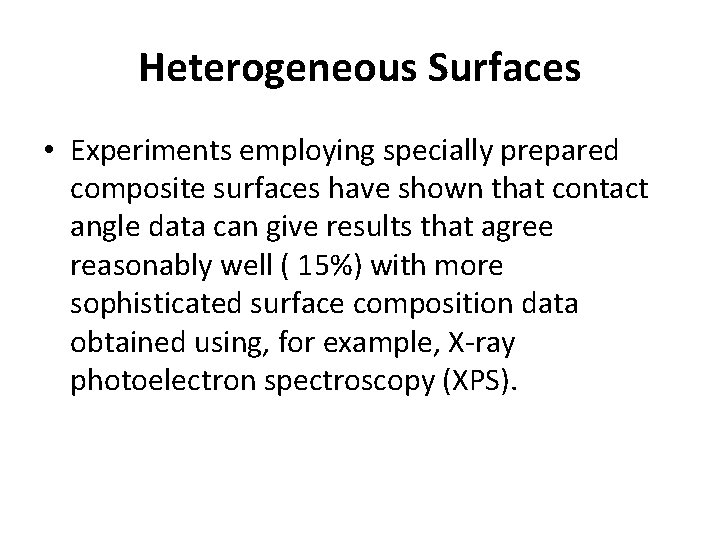
Heterogeneous Surfaces • Experiments employing specially prepared composite surfaces have shown that contact angle data can give results that agree reasonably well ( 15%) with more sophisticated surface composition data obtained using, for example, X-ray photoelectron spectroscopy (XPS).
 What is difference between abstract class and interface
What is difference between abstract class and interface What is difference between abstract class and interface
What is difference between abstract class and interface Interface and dialogue design
Interface and dialogue design Gui event types in java
Gui event types in java Which are not purely surface phenomena
Which are not purely surface phenomena Expressive interface
Expressive interface![[web user interface] [web user interface]](data:image/svg+xml,%3Csvg%20xmlns=%22http://www.w3.org/2000/svg%22%20viewBox=%220%200%20200%20200%22%3E%3C/svg%3E) [web user interface]
[web user interface] Uml interfaces are used to:
Uml interfaces are used to: Industrial interfaces
Industrial interfaces Expressive interface
Expressive interface Blueprint interfaces
Blueprint interfaces Which is not an objective of designing interfaces?
Which is not an objective of designing interfaces? Property management system interface
Property management system interface Similar to
Similar to Ims platform
Ims platform Contextual tools in hci
Contextual tools in hci Bts um
Bts um Expressive interfaces
Expressive interfaces Srs prototype outline
Srs prototype outline Team interfaces
Team interfaces Micros e interfaces
Micros e interfaces Why are user interfaces hard to implement
Why are user interfaces hard to implement Design heuristic
Design heuristic Tsesmelis
Tsesmelis Java gui for r
Java gui for r Interfaces inteligentes
Interfaces inteligentes Abstract classes in java
Abstract classes in java User interfaces design dc
User interfaces design dc Interface f
Interface f F-14
F-14 Communication interfaces in embedded systems
Communication interfaces in embedded systems Triangular pyramid faces edges vertices
Triangular pyramid faces edges vertices 12 edges and 6 faces
12 edges and 6 faces The relative lightness and darkness of surfaces.
The relative lightness and darkness of surfaces. Lines body cavities
Lines body cavities Normal inclined and oblique surfaces
Normal inclined and oblique surfaces Aircraft control surfaces and components
Aircraft control surfaces and components Aircraft control surfaces and components
Aircraft control surfaces and components Walking and working surfaces quiz
Walking and working surfaces quiz Ossification of tarsal bones
Ossification of tarsal bones Computer graphics
Computer graphics Walking on slippery surfaces
Walking on slippery surfaces How many vertices does a rectangular prism have
How many vertices does a rectangular prism have Subdivision surfaces in character animation
Subdivision surfaces in character animation To determine missing z values for a statistical surface
To determine missing z values for a statistical surface Explain reflection
Explain reflection Right and left border of heart
Right and left border of heart






![[web user interface] [web user interface]](https://slidetodoc.com/wp-content/uploads/2020/12/3515569_e80293ca9d02734a3126f3e8d1d1c3b7-300x225.jpg)