The TemperatureScanning PlugFlow Reactor SE Reactors Kinetic measurements











- Slides: 61

The Temperature-Scanning Plug-Flow Reactor SE Reactors

Kinetic measurements the way you always wanted them - FAST and EASY All of us who study reaction mechanisms using kinetics, all who test and evaluate catalysts, who need reliable rate expressions for reactor design or simulation, know how tedious and expensive it is to gather the necessary rate data. Well, here is the solution to these problems! SE Reactors

A New Kinetics Instrument The Temperature Scanning Reactor A TSR is not simply a laboratory reactor, it is a kinetics instrument, capable of establishing the rate parameters of a rate expression after less than a working day’s operation - temperature coefficients and all - for any candidate rate model you may wish to propose. SE Reactors

Why do Kinetic Studies ? Kinetic studies are essential to the understanding of reactions. They yield an appropriate reaction rate expression. If we know the rate expression, we can: l l l SE Reactors Design better catalyst formulations Draw inferences on the mechanism of the reaction Quantify the rate of reaction for process simulation Improve reactor control and design Look for optimum reaction conditions

Purpose of the TS-PFR The TS-PFR can be used to obtain “overall” reaction rates, under non-steady-state conditions, at commercially important temperatures and pressures. In much less time than by conventional means, we are able to determine: l l l Reaction rates. Reaction rate coefficients. Temperature dependence of rate coefficients. SE Reactors

Catalytic Reaction Rates Gas-phase catalytic reaction rates are usually measured in: l l isothermal, or, less commonly, adiabatic, plug-flow reactors. These two thermal regimes are difficult to implement experimentally, especially for highly exo/endothermic reactions. SE Reactors

Catalytic Reaction Rates With the introduction of the TS-PFR, we no longer need to operate under idealized and hard-to-implement thermal conditions. By operating in conformity with certain boundary conditions, the TS-PFR can determine an arbitrarily large number of reaction rates in one (say, 8 -hour) TSR “experiment”, without waiting for steady state to be established. SE Reactors

Mode of Operation of the TS-PFR l To do a “TSR RUN” in the TS-PFR one must: 1. Load the reactor with catalyst. 2. Establish a feed rate at an initial feed temperature. 3. Ramp the temperature of the feed at a selected rate. 4. Measure the output composition and temperature. 5. Terminate the ramp after a pre-selected time. l SE Reactors One “TSR EXPERIMENT” requires steps 2 to 5 to be repeated using exactly the same ramping procedure at a number of feed rates.

Mode of Operation of the TS-PFR It is important to realize that the data obtained by performing steps 1 to 5 only once i. e. , doing just one “TSR run”, does not lead to interpretable results. The TS algorithms must be applied to several such rampings, done under appropriate boundary conditions. Only data from such a ”TSR experiment” will allow the extraction of valid reaction rates. The rates obtained in this way are the same rates one would obtain from conventional isothermal experiments. SE Reactors

Boundary Conditions The boundary conditions which must be obeyed are simple to implement: 1. Exactly the same ramping sequence must be used in each run of a TS-PFR experiment 2. The reactor must be of uniform effectiveness along its length 3. Each run must begin with the reactor at the same condition throughout the bed (e. g. , at steady state for the initial conditions) 4. The temperature of the surroundings with which the reactor exchanges heat must be controlled in the same manner from run to run SE Reactors

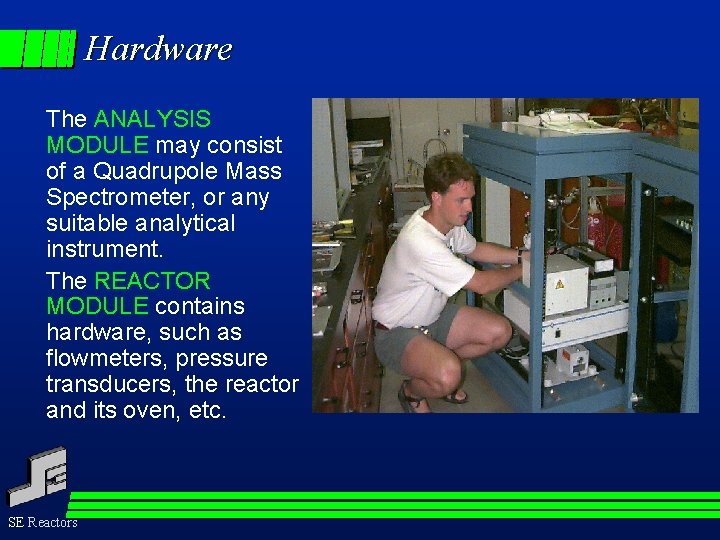
Hardware - General The TS-PFR consists of: Reactor Module Ì Analysis Module SE Reactors

Hardware The ANALYSIS MODULE may consist of a Quadrupole Mass Spectrometer, or any suitable analytical instrument. The REACTOR MODULE contains hardware, such as flowmeters, pressure transducers, the reactor and its oven, etc. SE Reactors

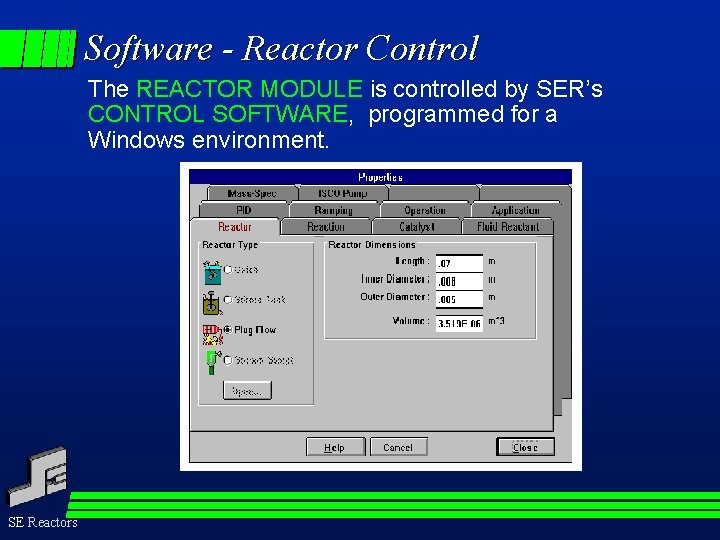
Software - Reactor Control The REACTOR MODULE is controlled by SER’s CONTROL SOFTWARE, programmed for a Windows environment. SE Reactors

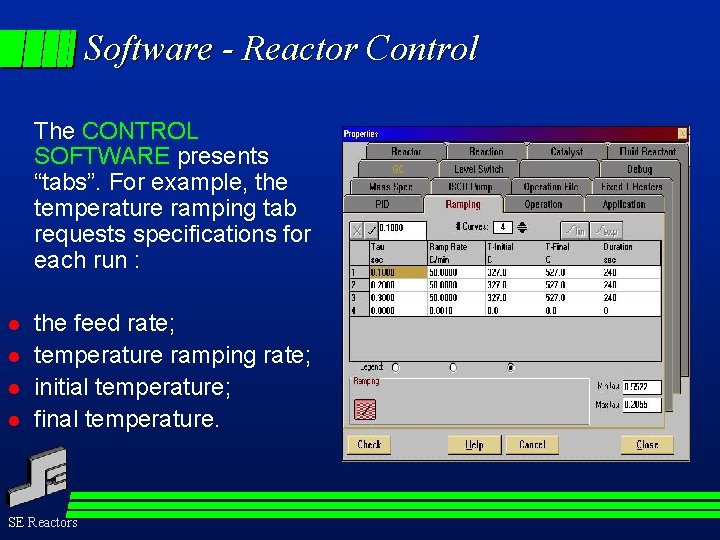
Software - Reactor Control The CONTROL SOFTWARE presents “tabs”. For example, the temperature ramping tab requests specifications for each run : l l the feed rate; temperature ramping rate; initial temperature; final temperature. SE Reactors

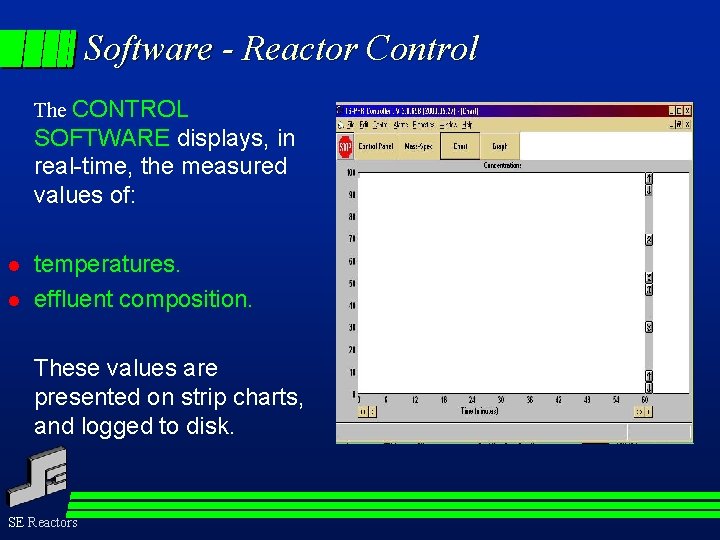
Software - Reactor Control The CONTROL SOFTWARE displays, in real-time, the measured values of: l l temperatures. effluent composition. These values are presented on strip charts, and logged to disk. SE Reactors

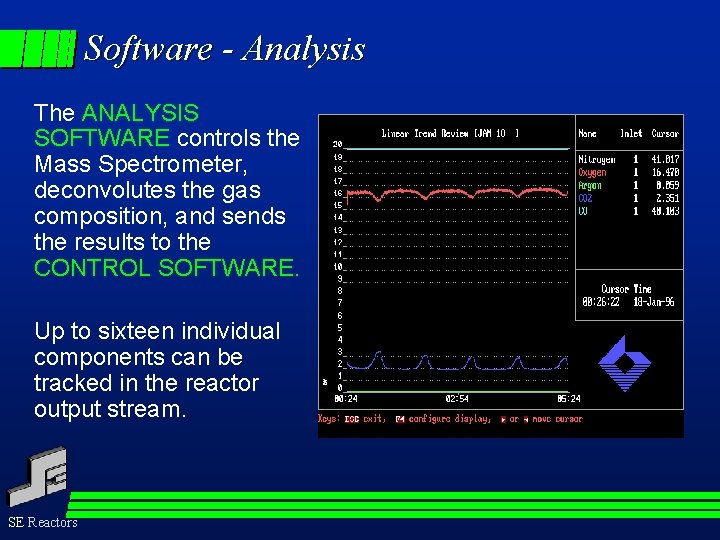
Software - Analysis The ANALYSIS SOFTWARE controls the Mass Spectrometer, deconvolutes the gas composition, and sends the results to the CONTROL SOFTWARE. Up to sixteen individual components can be tracked in the reactor output stream. SE Reactors

Software - Interpretation The “raw data” collected from the complete TSR experiment are collated and sent to the TS rate extraction program. There, rates are extracted at selected conditions and used to form X -T- r triplets. This “kinetic data” is then downloaded to a spreadsheet where the proposed rate expressions, r = f(X, T), can be fitted to the data. SE Reactors

Software - Interpretation If the rate expression is known, the spreadsheet “solver” is used to evaluate the rate parameters using the extracted X -T- r triplets of the kinetic data set, the known rate expression, and statistical tools. If the rate expression is not known, the kinetic data set is made available for fitting to candidate rate expressions. The success of the fit is judged on the basis of the statistics supplied by the solver, and by other tests appropriate to the system. SE Reactors

TSR Simulation SER has developed a Temperature-Scanning Reactor Simulator, capable of simulating a batch (TSBR), CSTR (TS-CSTR) and plug-flow (TS-PFR) reactor operating under temperature-scanning modes. All physical aspects are taken into account: i. e. , reactor materials, all heat transfer processes within the reactor system, heat of reaction, etc. Important note: there are no restrictions placed on heat transfer in the operation of a TS-PFR. We can simulate this point in detail and observe the effect on the reaction rates as they are extracted by the TSPFR algorithms. SE Reactors

Simulation - Heat Transfer Constants In the differential equations describing the behaviour of each of the TS reactors, there are constants (ki) in the heat balance equations which are calculated as functions of the real physical properties of the materials envisioned for the components of the simulated reactor. In a similar vein, all other physical aspects of the reactor system, such as mass of materials, pressure drop, etc. are included in the simulation using realistic values from established correlations. SE Reactors

Simulation - Rate Equation For simulation purposes, a “generic” Langmuir -Hinshelwood gas-solid catalytic reaction rate, with adsorption terms for both products and reactants, was used to model the kinetics. Each rate parameter was assumed to follow the Arrhenius temperature behaviour: SE Reactors

Simulation The following data is from such a simulation. This allows us to examine a wider range of conditions than are approachable in any one reaction system. In this way we examine, in one unified picture, the many phenomena which can arise in all systems, but all of which rarely arise at approachable conditions in any one system. SE Reactors

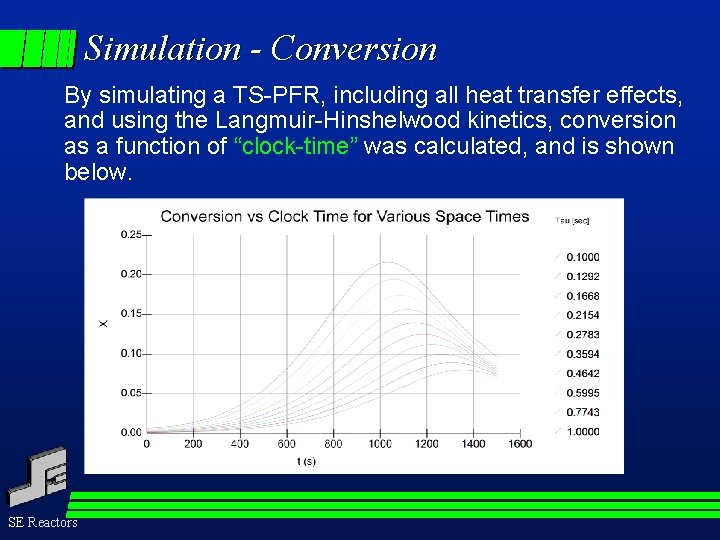
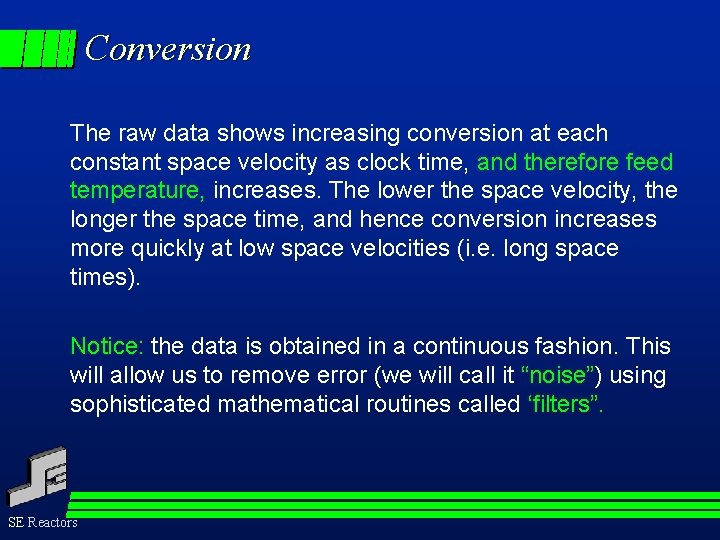
Simulation - Conversion By simulating a TS-PFR, including all heat transfer effects, and using the Langmuir-Hinshelwood kinetics, conversion as a function of “clock-time” was calculated, and is shown below. SE Reactors

Conversion The raw data shows increasing conversion at each constant space velocity as clock time, and therefore feed temperature, increases. The lower the space velocity, the longer the space time, and hence conversion increases more quickly at low space velocities (i. e. long space times). Notice: the data is obtained in a continuous fashion. This will allow us to remove error (we will call it “noise”) using sophisticated mathematical routines called ‘filters”. SE Reactors

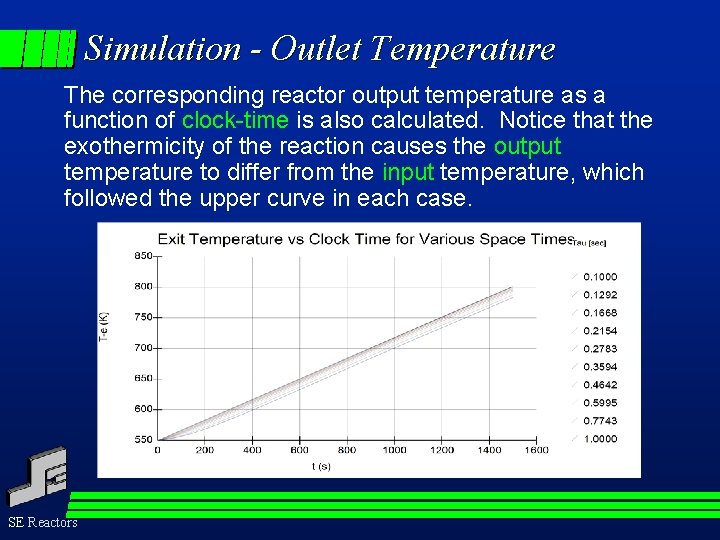
Simulation - Outlet Temperature The corresponding reactor output temperature as a function of clock-time is also calculated. Notice that the exothermicity of the reaction causes the output temperature to differ from the input temperature, which followed the upper curve in each case. SE Reactors

Simulation - Outlet Temperature The reactor output temperature is a function of clock-time due to: a) the temperature ramping, and b) the exothermicity of the model reaction. The output temperature will differ from reaction to reaction and confirms the non-ideality of the reactor. The theory of TSR operation describes how this non-ideality can be removed so that correct reaction rates can be calculated from TSR data. SE Reactors

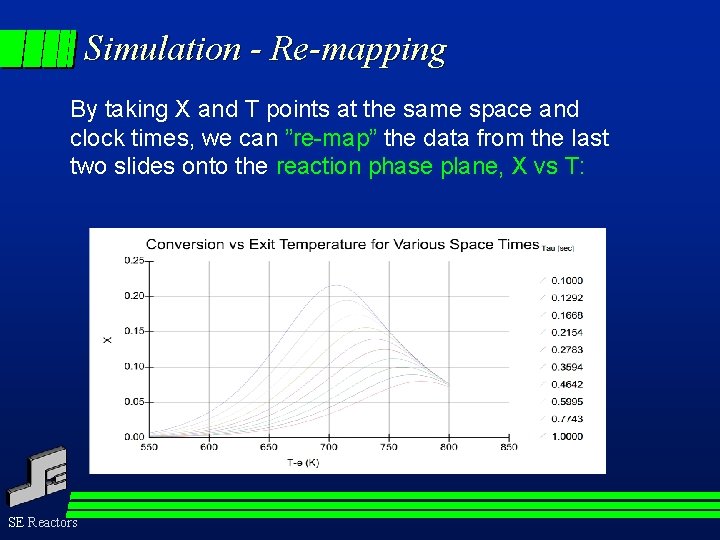
Simulation - Re-mapping By taking X and T points at the same space and clock times, we can ”re-map” the data from the last two slides onto the reaction phase plane, X vs T: SE Reactors

Re-mapping It is in this plane that the presence of non-kinetic influences is detected. Catalyst aging, diffusion effects and any such distorting influences are readily discovered by this re-mapping of the raw data. In most cases these effects can be quantified by pursuing an appropriate experimental program. The essence of data treatment and of the understanding made available by TSR experimentation lies in such re-mappings of the data collected. SE Reactors

Simulation – 3 D - mapping After just ten runs the experimental data presents enough information to delineate a smooth (T, t, X) surface allowing for accurate interpolation. SE Reactors

3 D - mapping The curves on the X, T plane are contour lines from this three dimensional surface. With ten curves or so there is usually enough data to delineate the full (T, t, X) surface. This is the source of the unlimited data available from a TSR: the data are obtained in continuous fashion allowing sophisticated two-dimensional filters to construct a smooth surface. The smooth surface in turn allows any point within its confines to be accessed. SE Reactors

Surveying the “Reaction Surface” We now see that there exists a “reaction surface” which we “survey” using a chemical reactor. In the case of an isothermal PFR, one can measure any point on this surface, but in the case of a TS-PFR one must follow prescribed “traverses” which restricted movement during the survey. SE Reactors

Surveying the “Reaction Surface” by Isothermal Operation The conventional isothermal method of surveying involves taking a small set of readings, at isolated points, along a few isothermal traverses. Each of these data points represents an independent measurement, with its own error. In conventional studies the 10 to 20 points collected in this way are used to estimate the shape of this surface and then to fit a rate equation which reproduces this shape. SE Reactors

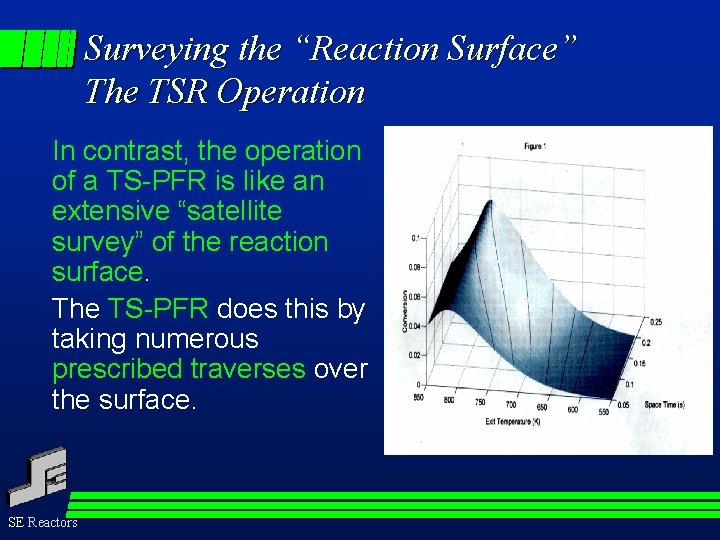
Surveying the “Reaction Surface” The TSR Operation In contrast, the operation of a TS-PFR is like an extensive “satellite survey” of the reaction surface. The TS-PFR does this by taking numerous prescribed traverses over the surface. SE Reactors

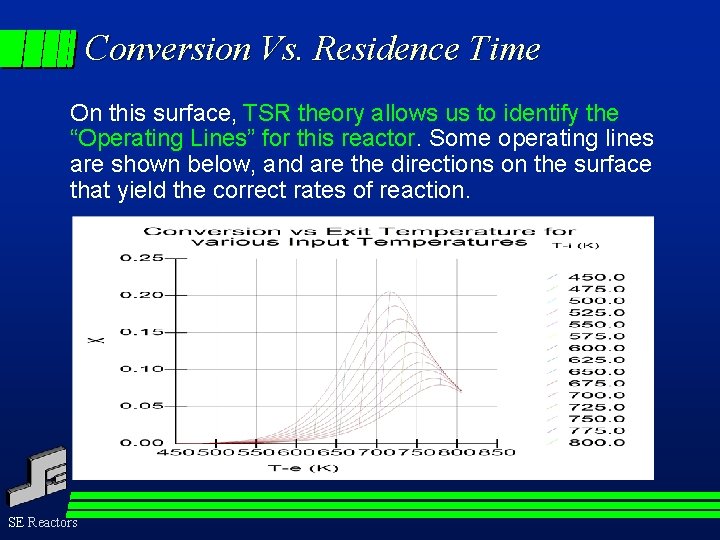
Conversion Vs. Residence Time On this surface, TSR theory allows us to identify the “Operating Lines” for this reactor. Some operating lines are shown below, and are the directions on the surface that yield the correct rates of reaction. SE Reactors

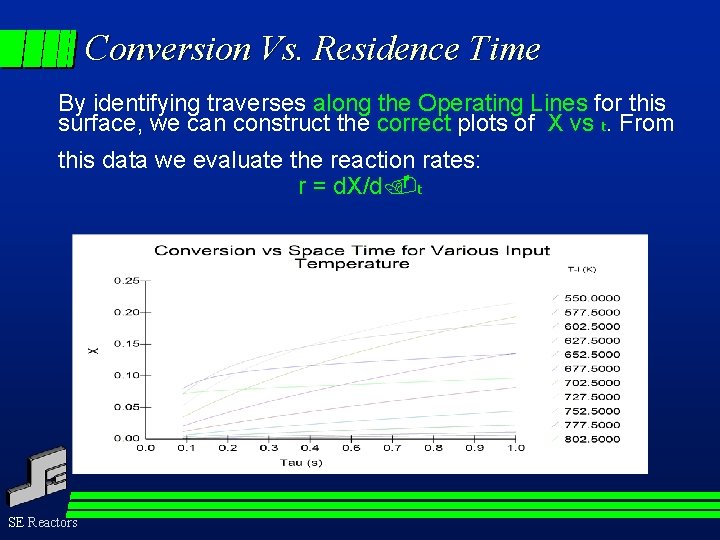
Conversion Vs. Residence Time By identifying traverses along the Operating Lines for this surface, we can construct the correct plots of X vs t. From this data we evaluate the reaction rates: r = d. X/d t SE Reactors

Extraction of Rates To collect the set of (X -T - r) triplets required for fitting to a rate expression we must therefore: l spline the discrete X-t data using a suitable spline function. l evaluate d. X/dt at the desired values of X and t. l read the outlet temperature at the corresponding X and t from the (T, X)t curves. SE Reactors

The Triplets In this way we form the (X, T, r) triplets necessary for the fitting of a rate expression. Each (X - T - r ) triplet collected in this way contains all the values necessary to fit a rate expression. We can collect an arbitrarily large number of such triplets from each experiment. With these we can proceed to search for the appropriate rate expression, to establish its rate parameters, or to examine its behaviour visually. SE Reactors

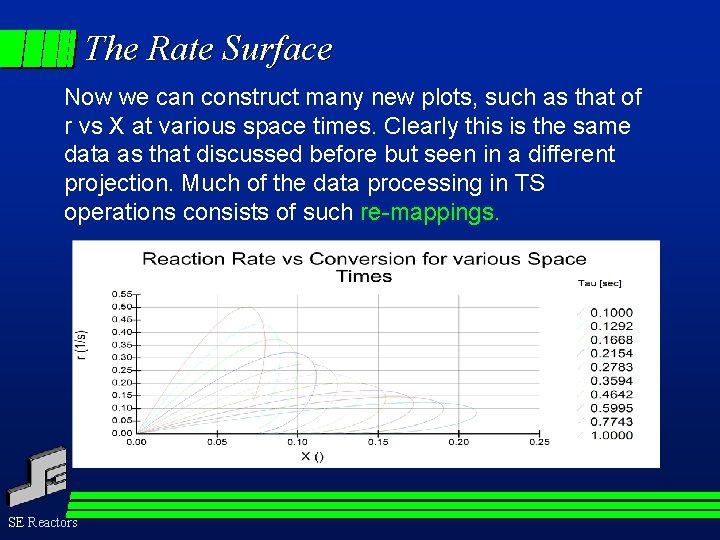
The Rate Surface Now we can construct many new plots, such as that of r vs X at various space times. Clearly this is the same data as that discussed before but seen in a different projection. Much of the data processing in TS operations consists of such re-mappings. SE Reactors

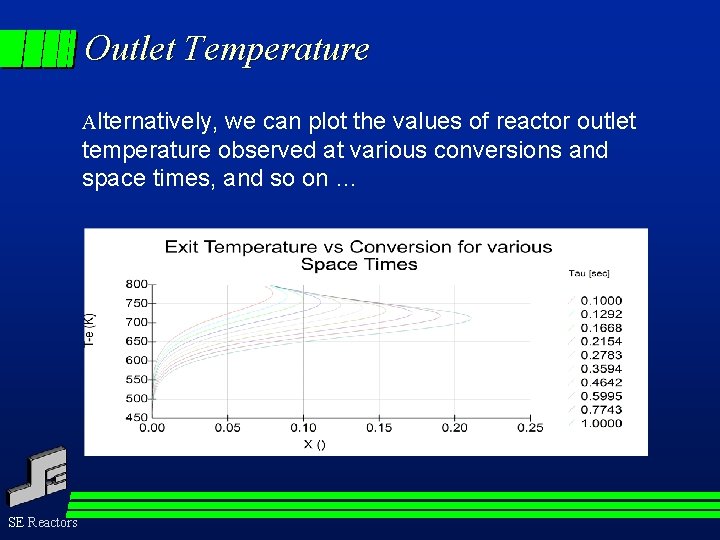
Outlet Temperature Alternatively, we can plot the values of reactor outlet temperature observed at various conversions and space times, and so on … SE Reactors

Re-Mappings In fact, each data point has associated with it the dimensions of è è è Conversion Inlet Temperature Outlet Temperature Space Time Clock Time Reaction Rate We can therefore examine TSR data in a large variety of 2 D and 3 D presentations. SE Reactors

The Rich Harvest of Rates As many of the (X -T - r) triplets as we may wish to have are made available by the procedure of defining a smooth surface using the dense mesh of raw experimental data. We now proceed to “sieve out” appropriate sets of data for model-fitting, or any other purpose. For example, we could select sets of isothermal (constant T) or sets of isokinetic (constant r) X-T-r data. SE Reactors

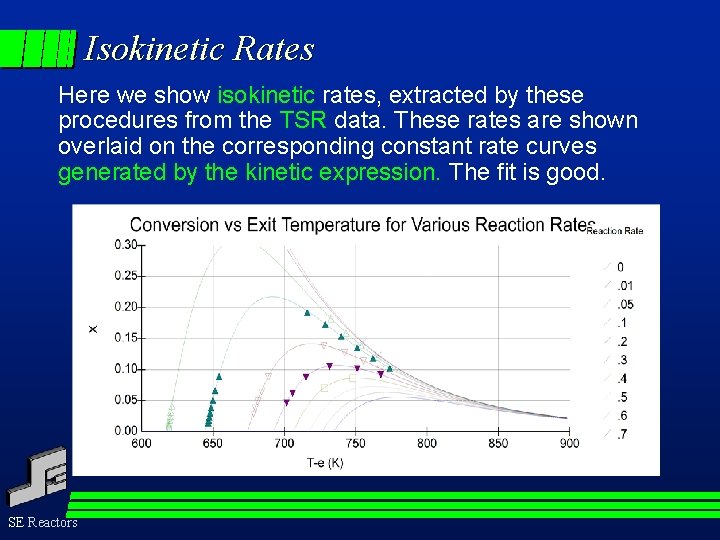
Isokinetic Rates Here we show isokinetic rates, extracted by these procedures from the TSR data. These rates are shown overlaid on the corresponding constant rate curves generated by the kinetic expression. The fit is good. SE Reactors

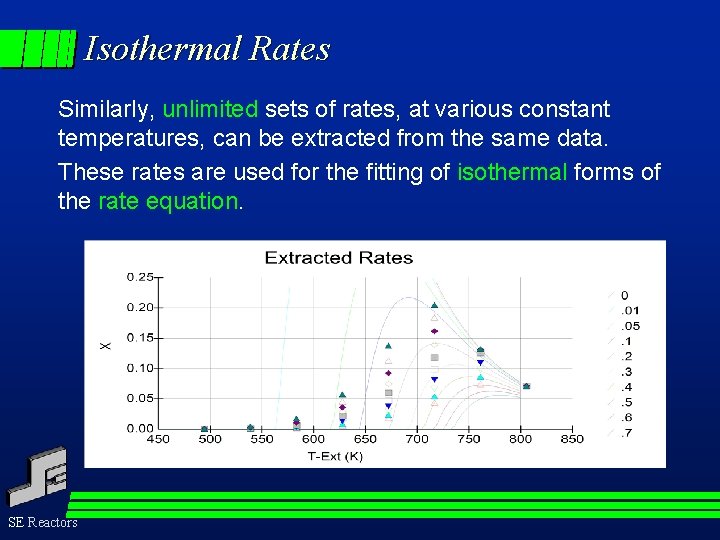
Isothermal Rates Similarly, unlimited sets of rates, at various constant temperatures, can be extracted from the same data. These rates are used for the fitting of isothermal forms of the rate equation. SE Reactors

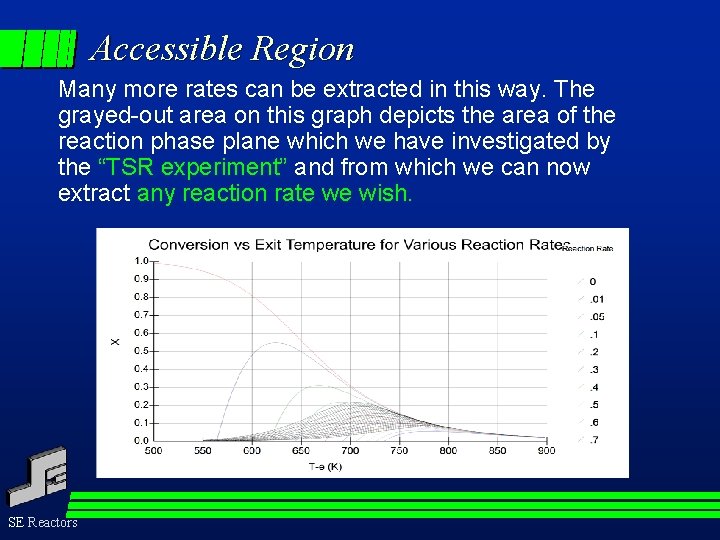
Accessible Region Many more rates can be extracted in this way. The grayed-out area on this graph depicts the area of the reaction phase plane which we have investigated by the “TSR experiment” and from which we can now extract any reaction rate we wish. SE Reactors

Range of Accessibility The grayed-out area presents all the data that can be obtained using this PFR. l l The lower bound is defined by a run at a space velocity which causes maximum tolerable pressure drop through the catalyst bed. The upper bound is at a space velocity which is at a Reynolds number on the brink of transition to laminar flow. Between these two limits lies all of the performance space accessible to this plug flow reactor, for this reaction, regardless of the mode of operation. SE Reactors

CO Oxidation Experimental results, using the real TS-PFR described previously, were gathered in a study of the catalytic oxidation of carbon monoxide, performed on a proprietary automotive catalyst. The raw data and results are presented in the following slides. SE Reactors

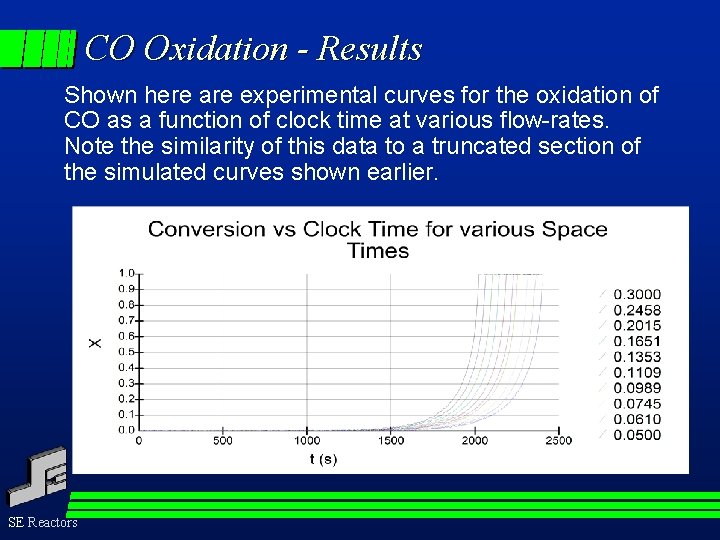
CO Oxidation - Results Shown here are experimental curves for the oxidation of CO as a function of clock time at various flow-rates. Note the similarity of this data to a truncated section of the simulated curves shown earlier. SE Reactors

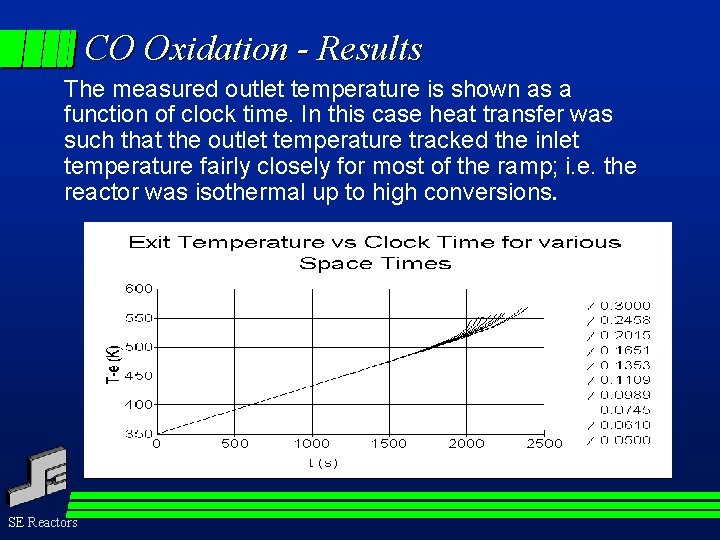
CO Oxidation - Results The measured outlet temperature is shown as a function of clock time. In this case heat transfer was such that the outlet temperature tracked the inlet temperature fairly closely for most of the ramp; i. e. the reactor was isothermal up to high conversions. SE Reactors

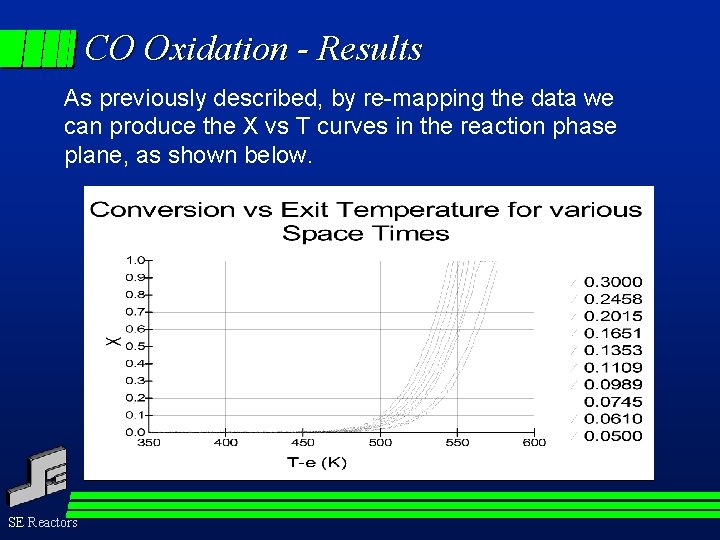
CO Oxidation - Results As previously described, by re-mapping the data we can produce the X vs T curves in the reaction phase plane, as shown below. SE Reactors

CO Oxidation - Interpretation The experimental conversion vs. space time data must be smoothed, or filtered, in order to extract rates successfully, since numerical derivatives of d. X/dt will have to be calculated. The taking of point to point differentials from noisy data amplifies the noise present in the original data and will distort the interpretation. Within the TSR interpretation software, several datafiltering techniques have been made available. Many more filters are available in the literature. SE Reactors

Filtering The many mathematical filters available are designed to deal with specific types of noise, each has special merit in particular circumstances l FFT smoothing in the frequency domain; l Savitsky-Golay filtering; and l 2 -Dimensional “surface smoothing” using least - squares splines. Are available in the TSR software. Once the data have been properly “filtered”, the interpretation techniques described earlier can be applied. SE Reactors

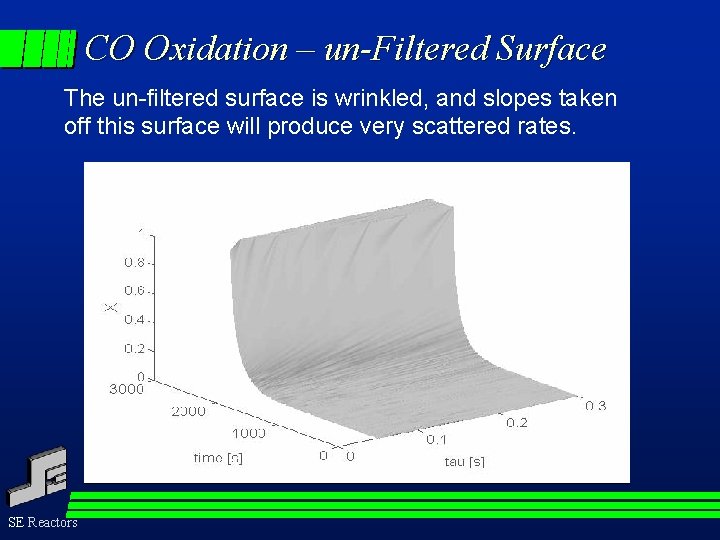
CO Oxidation – un-Filtered Surface The un-filtered surface is wrinkled, and slopes taken off this surface will produce very scattered rates. SE Reactors

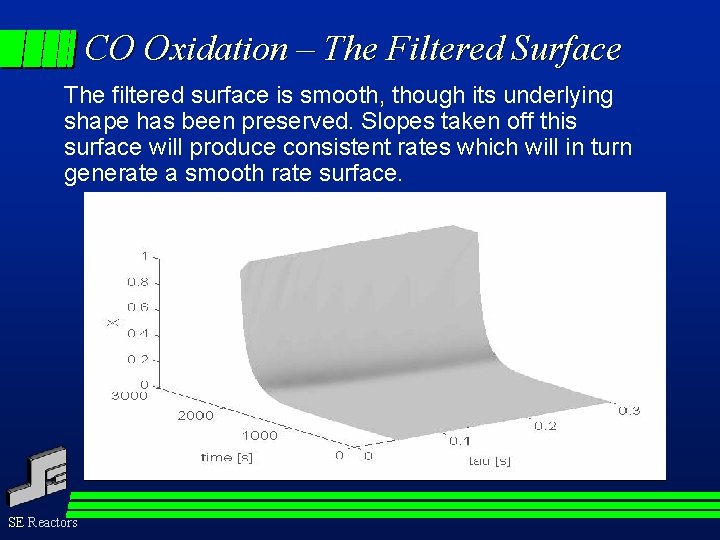
CO Oxidation – The Filtered Surface The filtered surface is smooth, though its underlying shape has been preserved. Slopes taken off this surface will produce consistent rates which will in turn generate a smooth rate surface. SE Reactors

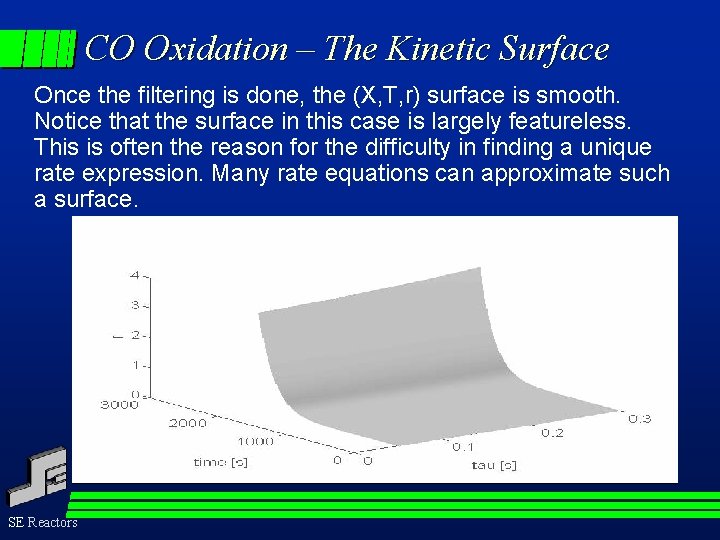
CO Oxidation – The Kinetic Surface Once the filtering is done, the (X, T, r) surface is smooth. Notice that the surface in this case is largely featureless. This is often the reason for the difficulty in finding a unique rate expression. Many rate equations can approximate such a surface. SE Reactors

CO Oxidation – The Rate Fitting Although this particular kinetic surface is featureless, it is still generated by a unique rate expression. In order to identify this expression we need to have as much of the surface surveyed as possible, and it must be as smooth as possible. These are the reasons why the TSR produces results which are greatly superior to those obtained by traditional isothermal experimentation. è è SE Reactors The TSR produces much more data; The data can be smoothed in a rational way.

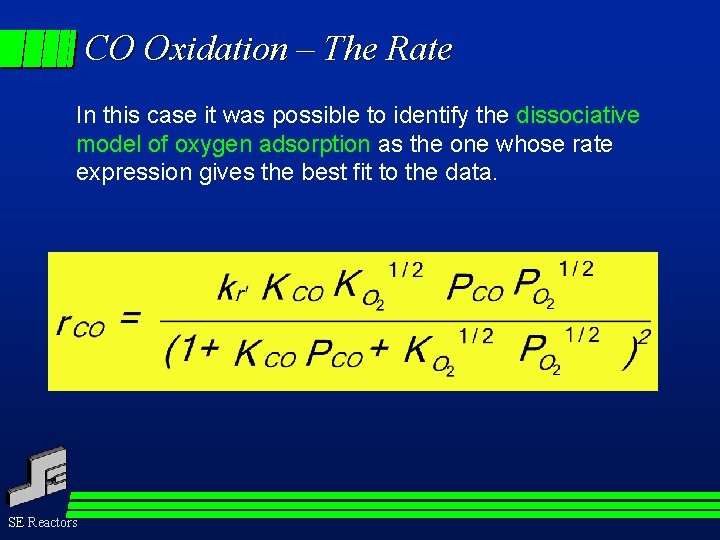
CO Oxidation – The Rate In this case it was possible to identify the dissociative model of oxygen adsorption as the one whose rate expression gives the best fit to the data. SE Reactors

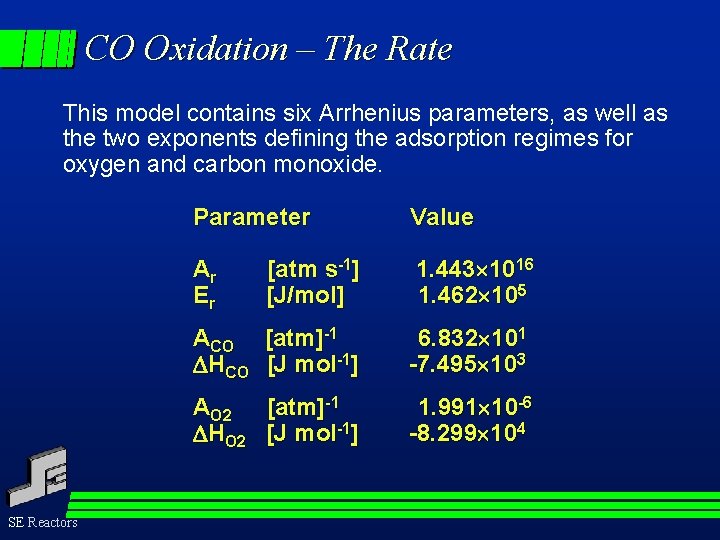
CO Oxidation – The Rate This model contains six Arrhenius parameters, as well as the two exponents defining the adsorption regimes for oxygen and carbon monoxide. SE Reactors Parameter Value Ar Er [atm s-1] [J/mol] 1. 443 1016 1. 462 105 ACO [atm]-1 HCO [J mol-1] 6. 832 101 -7. 495 103 AO 2 [atm]-1 HO 2 [J mol-1] 1. 991 10 -6 -8. 299 104

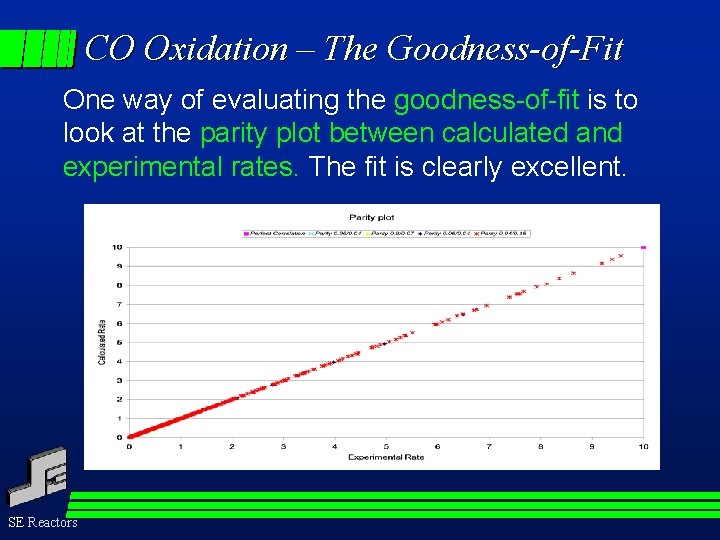
CO Oxidation – The Goodness-of-Fit One way of evaluating the goodness-of-fit is to look at the parity plot between calculated and experimental rates. The fit is clearly excellent. SE Reactors

How Robust is this Procedure ? If we obey the boundary conditions, then: – – heat transfer between system components ramping rates pressure drop, and the absence of a thermal steady state will not affect the TS algorithm. We will always extract the correct reaction rates. SE Reactors

Conclusions - What are the Benefits ? Compared to a conventional reactor operating at isothermal conditions, an automated TS-PFR gives: l l a very large number of “filtered” reaction rates, in a very short time. As a consequence, data are easier and cheaper to acquire. One need no longer be satisfied with the limited information available at a “standard test” condition. SE Reactors

The Temperature-Scanning Plug. Flow Reactor The Kinetics Instrument SE Reactors