Reactor physics Reactor training course Institut fr Kernchemie










![Inhour equation Für TRIGA 1 dollar [$] = = 0. 0073 , 1 cent Inhour equation Für TRIGA 1 dollar [$] = = 0. 0073 , 1 cent](https://slidetodoc.com/presentation_image_h/8439376dabcbd64fc75ca4441331dd1c/image-36.jpg)



- Slides: 45

Reactor physics Reactor training course Institut für Kernchemie www. kernchemie. uni-mainz. de

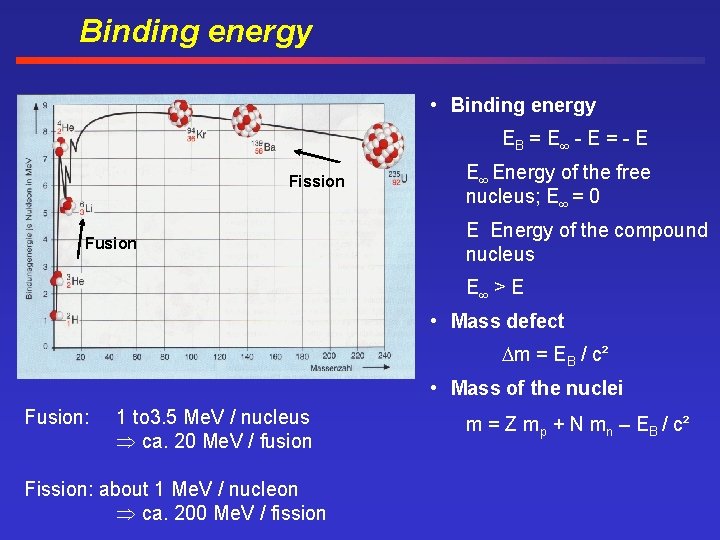
Binding energy • Binding energy EB = E - E = - E Fission Fusion E Energy of the free nucleus; E = 0 E Energy of the compound nucleus E > E • Mass defect m = EB / c² • Mass of the nuclei Fusion: 1 to 3. 5 Me. V / nucleus ca. 20 Me. V / fusion Fission: about 1 Me. V / nucleon ca. 200 Me. V / fission m = Z mp + N mn – EB / c²

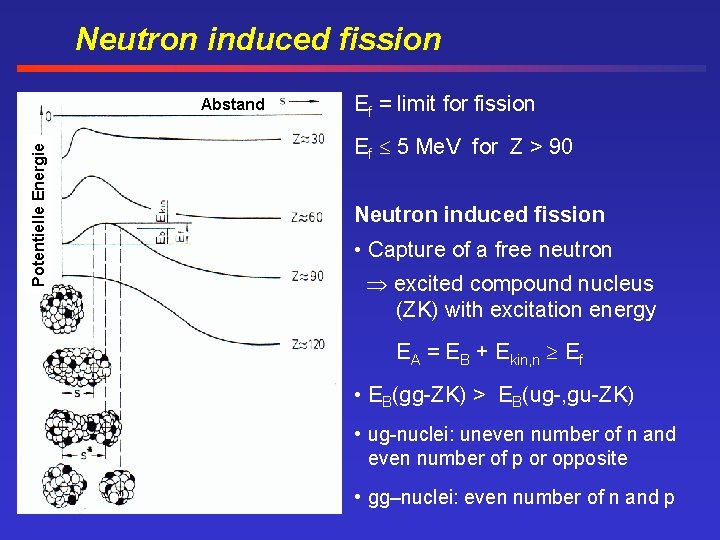
Neutron induced fission Potentielle Energie Abstand Ef = limit for fission Ef 5 Me. V for Z > 90 Neutron induced fission • Capture of a free neutron excited compound nucleus (ZK) with excitation energy EA = EB + Ekin, n Ef • EB(gg-ZK) > EB(ug-, gu-ZK) • ug-nuclei: uneven number of n and even number of p or opposite • gg–nuclei: even number of n and p

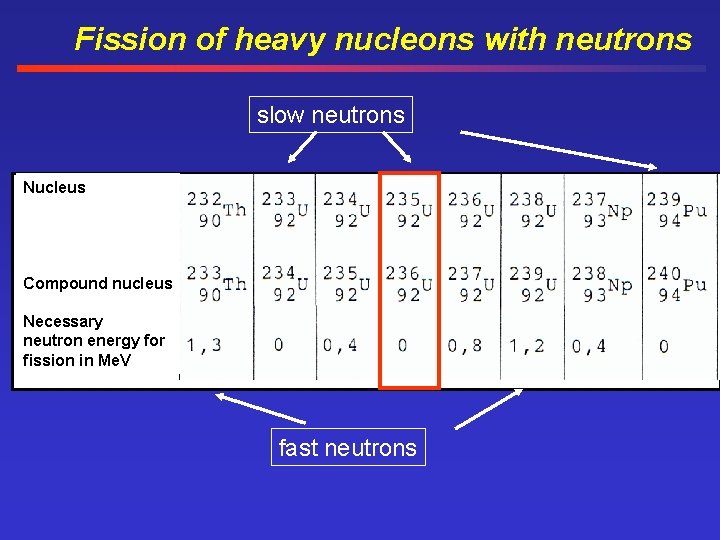
Fission of heavy nucleons with neutrons slow neutrons Nucleus Compound nucleus Necessary neutron energy for fission in Me. V fast neutrons

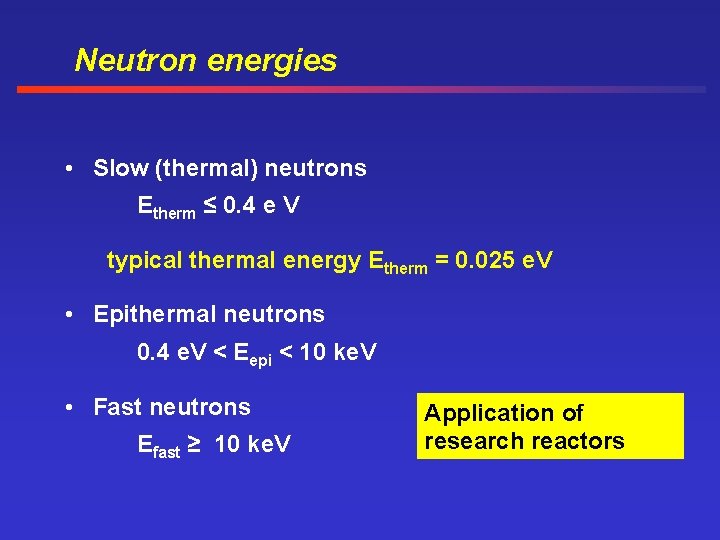
Neutron energies • Slow (thermal) neutrons Etherm ≤ 0. 4 e V typical thermal energy Etherm = 0. 025 e. V • Epithermal neutrons 0. 4 e. V < Eepi < 10 ke. V • Fast neutrons Efast ≥ 10 ke. V Application of research reactors

Uranium 1 g 238 U • 20 spontaneous fissions per hour (tunnel effect) • 106 times more -decays Natural Uranium • 0. 72 % 235 U • 99. 28 % 238 U Enrichment of 235 U • Power reactors: 3 -4% • Research reactors: 20 % (LEU) > 20 % (HEU) fuel development

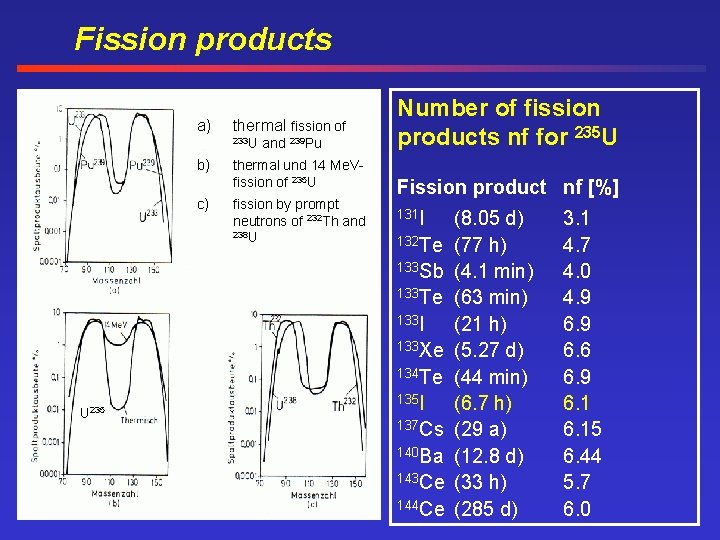
Fission products a) thermal fission of 233 U b) c) and 239 Pu thermal und 14 Me. Vfission of 235 U fission by prompt neutrons of 232 Th and 238 U Number of fission products nf for 235 U Fission product nf [%] 131 I 132 Te 133 Sb 133 Te 133 I 133 Xe 134 Te U 235 135 I 137 Cs 140 Ba 143 Ce 144 Ce (8. 05 d) (77 h) (4. 1 min) (63 min) (21 h) (5. 27 d) (44 min) (6. 7 h) (29 a) (12. 8 d) (33 h) (285 d) 3. 1 4. 7 4. 0 4. 9 6. 6 6. 9 6. 15 6. 44 5. 7 6. 0

Activation • Structural components of the reactor Aluminum Stainless steel Concrete • Air, water

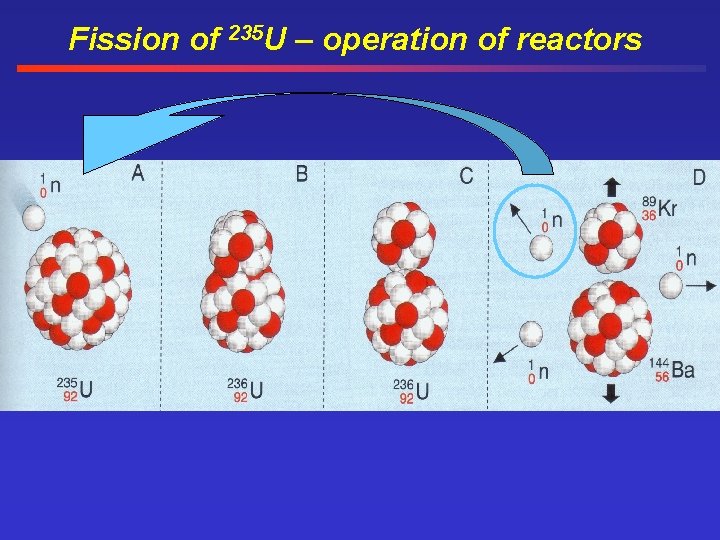
Fission of 235 U – operation of reactors

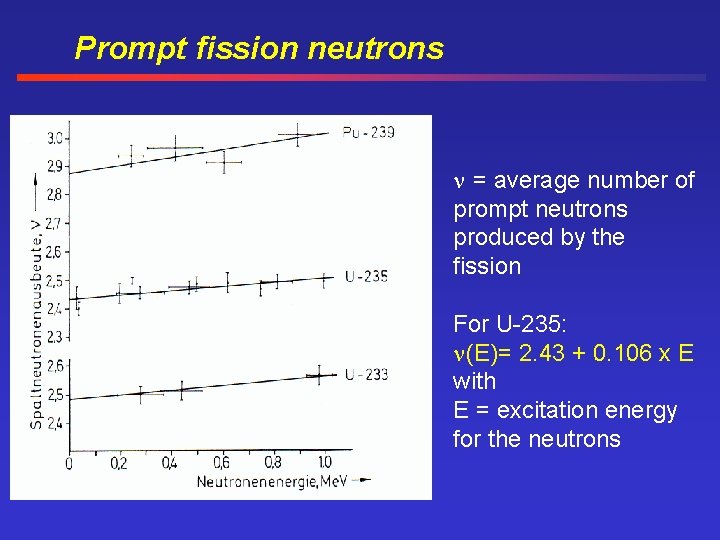
Prompt fission neutrons = average number of prompt neutrons produced by the fission For U-235: (E)= 2. 43 + 0. 106 x E with E = excitation energy for the neutrons

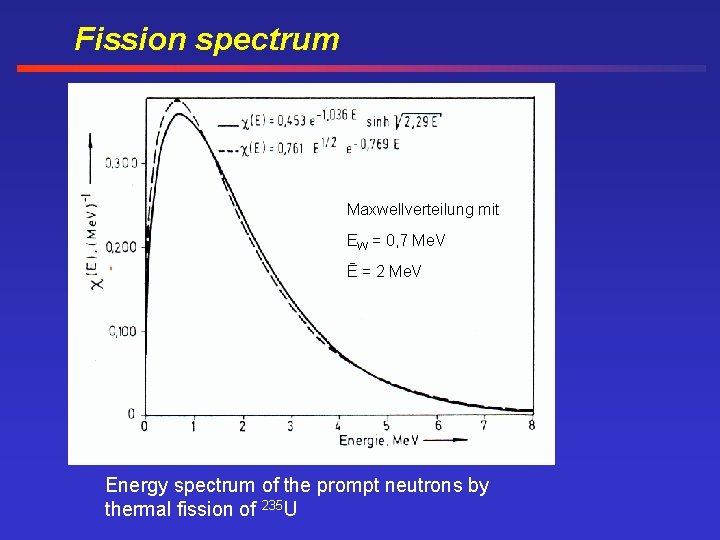
Fission spectrum Maxwellverteilung mit EW = 0, 7 Me. V Ē = 2 Me. V Energy spectrum of the prompt neutrons by thermal fission of 235 U

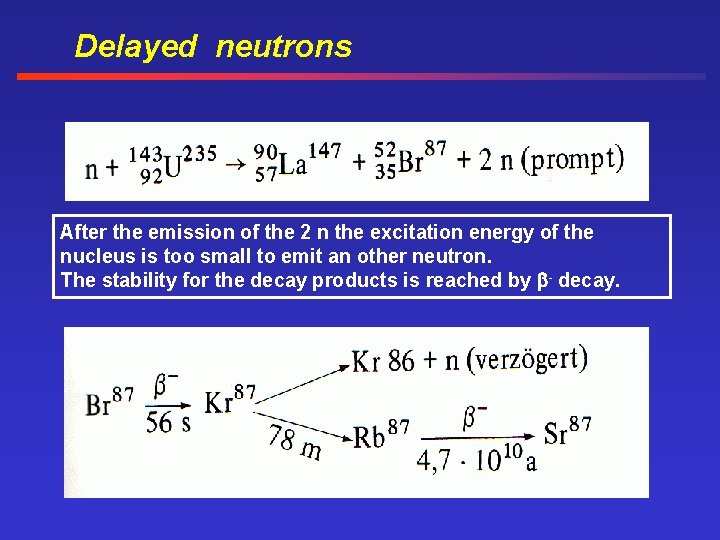
Delayed neutrons After the emission of the 2 n the excitation energy of the nucleus is too small to emit an other neutron. The stability for the decay products is reached by - decay.

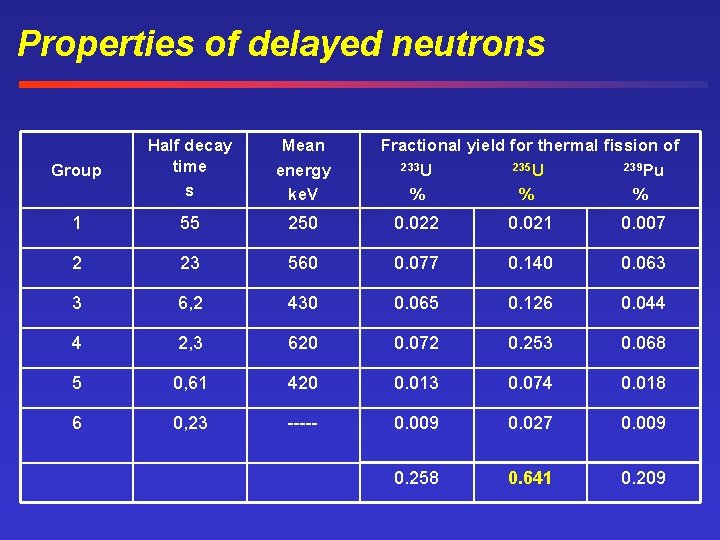
Properties of delayed neutrons Group Half decay time s Mean energy ke. V Fractional yield for thermal fission of 233 U 235 U 239 Pu % % % 1 55 250 0. 022 0. 021 0. 007 2 23 560 0. 077 0. 140 0. 063 3 6, 2 430 0. 065 0. 126 0. 044 4 2, 3 620 0. 072 0. 253 0. 068 5 0, 61 420 0. 013 0. 074 0. 018 6 0, 23 ----- 0. 009 0. 027 0. 009 0. 258 0. 641 0. 209

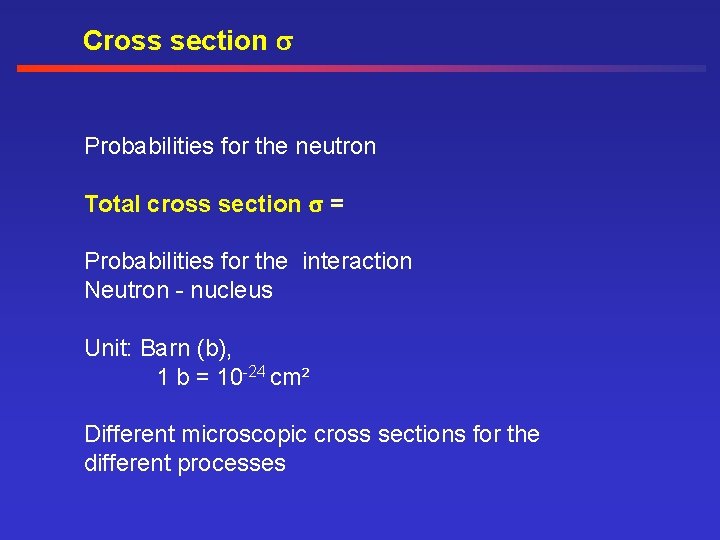
Cross section Probabilities for the neutron Total cross section = Probabilities for the interaction Neutron - nucleus Unit: Barn (b), 1 b = 10 -24 cm² Different microscopic cross sections for the different processes

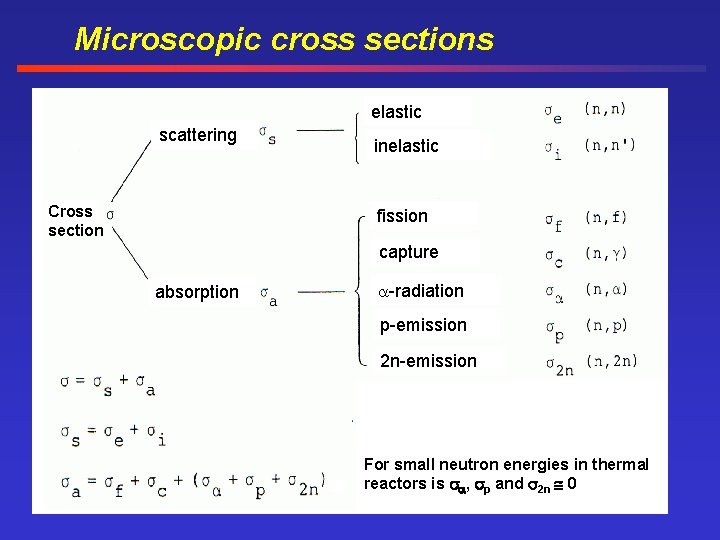
Microscopic cross sections elastic scattering Cross section inelastic fission capture absorption -radiation p-emission 2 n-emission For small neutron energies in thermal reactors is , p and 2 n 0

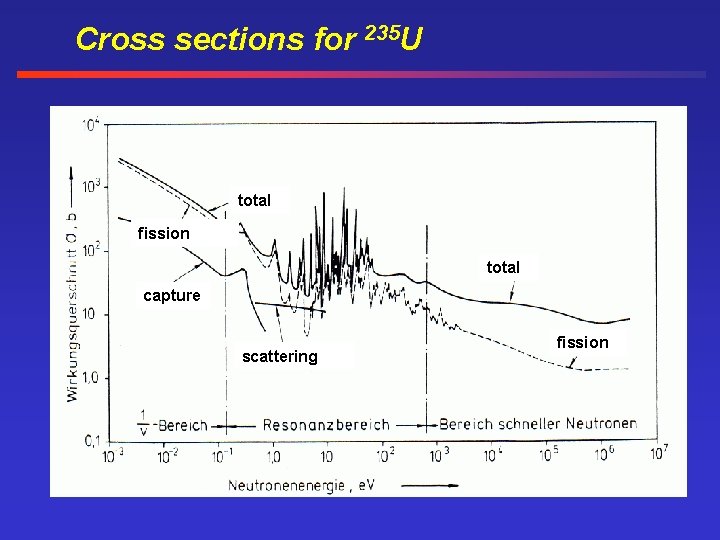
Cross sections for 235 U total fission total capture scattering fission

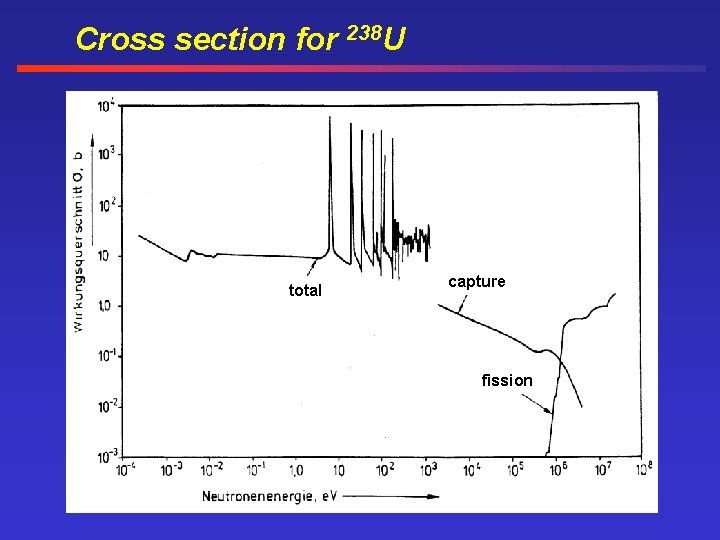
Cross section for 238 U total capture fission

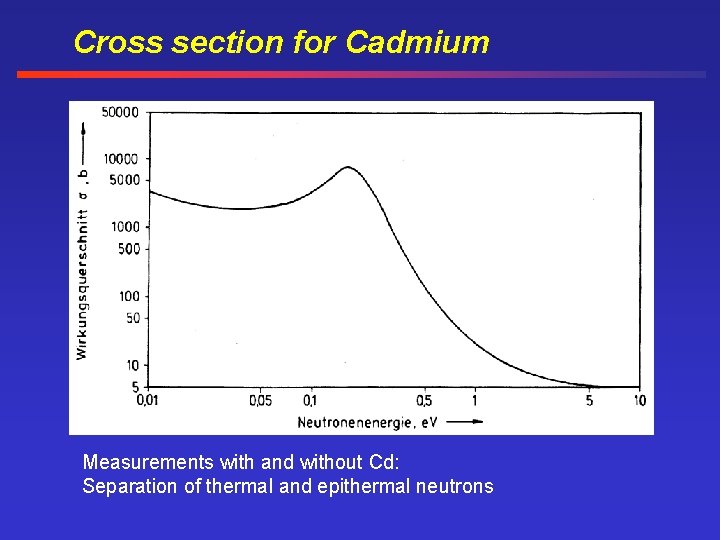
Cross section for Cadmium Measurements with and without Cd: Separation of thermal and epithermal neutrons

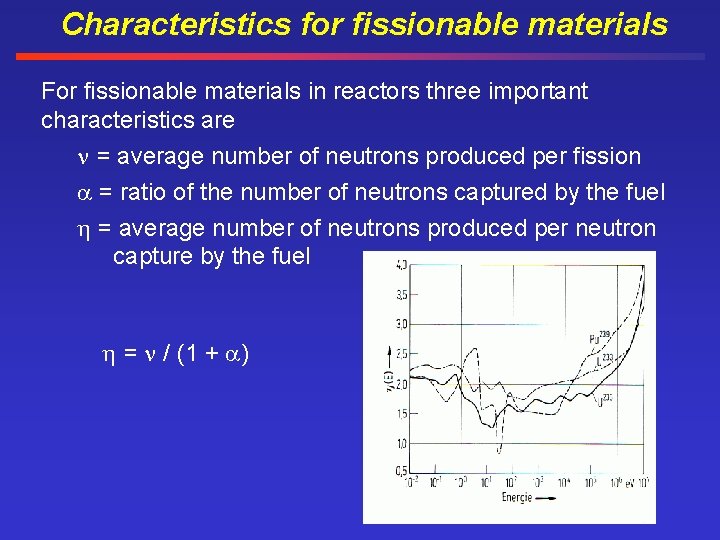
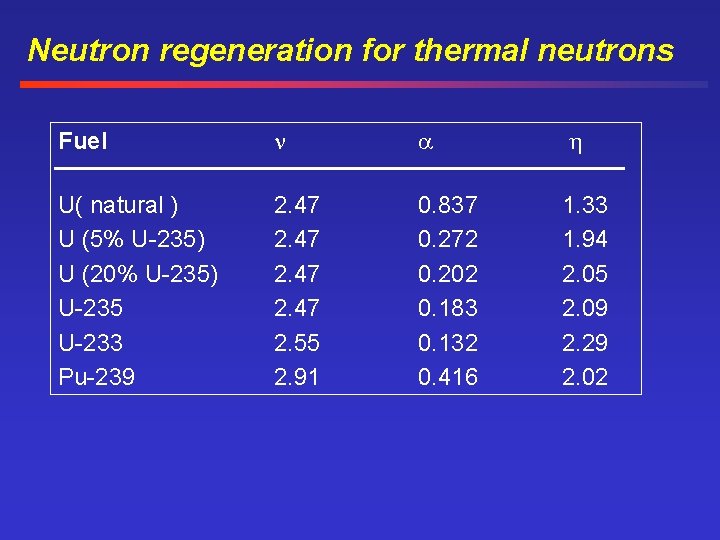
Characteristics for fissionable materials For fissionable materials in reactors three important characteristics are = average number of neutrons produced per fission = ratio of the number of neutrons captured by the fuel = average number of neutrons produced per neutron capture by the fuel = / (1 + )

Neutron regeneration for thermal neutrons Fuel U( natural ) U (5% U-235) U (20% U-235) U-235 U-233 Pu-239 2. 47 2. 55 2. 91 0. 837 0. 272 0. 202 0. 183 0. 132 0. 416 1. 33 1. 94 2. 05 2. 09 2. 29 2. 02

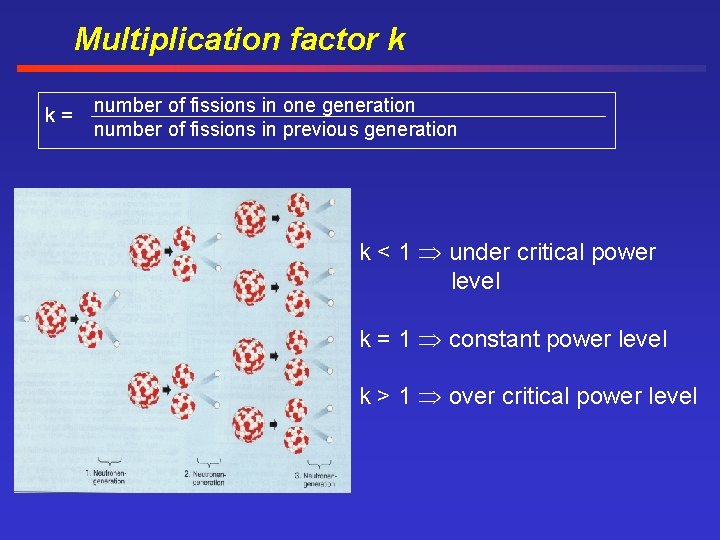
Multiplication factor k k= number of fissions in one generation number of fissions in previous generation k < 1 under critical power level k = 1 constant power level k > 1 over critical power level

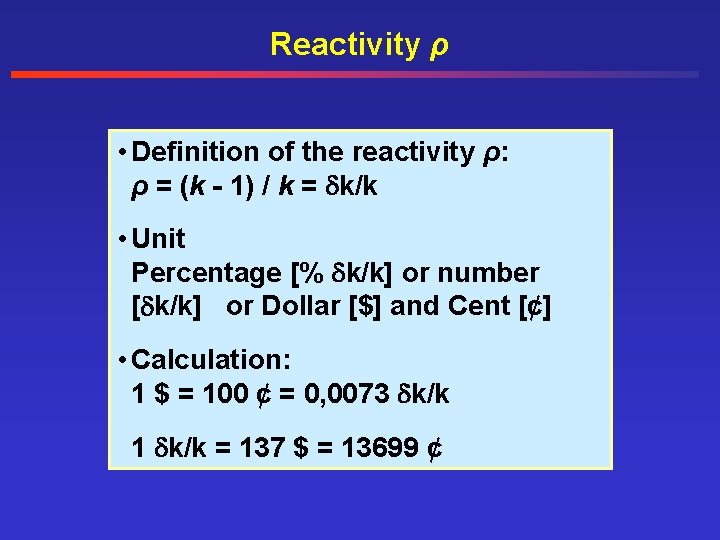
Reactivity ρ • Definition of the reactivity ρ: ρ = (k - 1) / k = k/k • Unit Percentage [% k/k] or number [ k/k] or Dollar [$] and Cent [¢] • Calculation: 1 $ = 100 ¢ = 0, 0073 k/k 1 k/k = 137 $ = 13699 ¢

Reaktivität ρ < 0 under critical power level = 0 constant power level > 0 over critical power level

Four – factor formula For large reactors an infinite multiplication factor is defined k = . . p. f = number of neutrons produced per neutron absorbed in the fuel = fast fission factor, a correction factor to take into account the fact that some fissions will be produced by fast neutrons p = resonant escape probability, the probability that a neutron will escape from capture while it is being slowed through the resonance energy range (approximately 1 to 100 e. V). f = thermal utilization, the fraction of thermal neutrons which are absorbed in in the fuel

Four – factor formula - examples Homogenous mixture of natural uranium and graphite, both being powder: Number of neutrons produced per absorbed neutron = 1. 33 Fast fission factor 1. 0, Product of resonant escape probability p and thermal utilization f: p. f 0. 6 k = . . p. f = 1. 33. 1. 0. 6 = 0. 8 Impossible to use such a combination in a reactor If the uranium is used in rods with diameters of 1 to 2 inch in a matrix of solid graphite (heterogeneous system) p is increased and the product p. f 0. 8 Fast fission factor 1. 03 k = 1. 33. 1. 03. 0. 8 = 1. 09 Possible to construct such a reactor

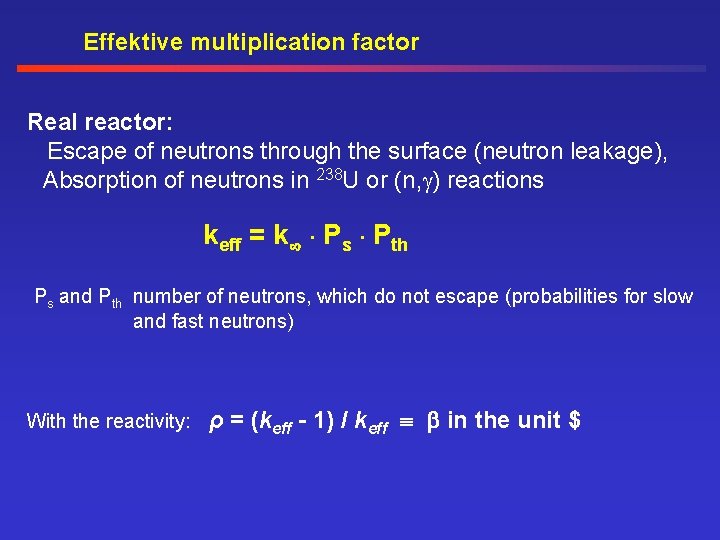
Effektive multiplication factor Real reactor: Escape of neutrons through the surface (neutron leakage), Absorption of neutrons in 238 U or (n, ) reactions keff = k . Ps. Pth Ps and Pth number of neutrons, which do not escape (probabilities for slow and fast neutrons) With the reactivity: ρ = (keff - 1) / keff in the unit $

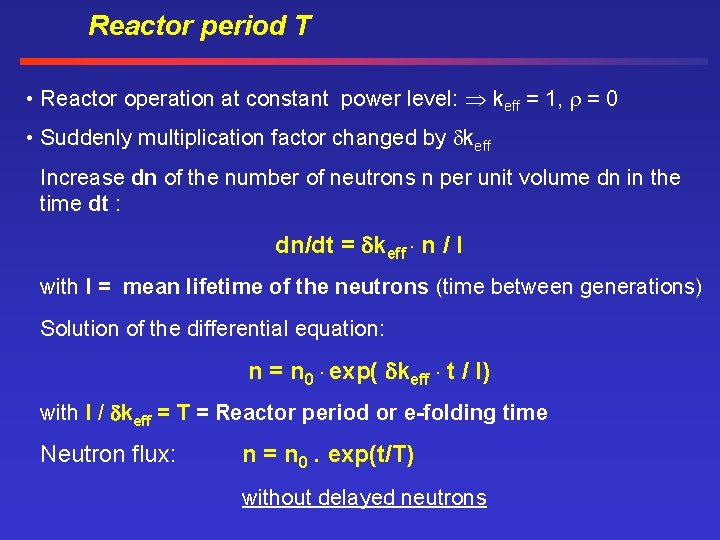
Reactor period T • Reactor operation at constant power level: keff = 1, = 0 • Suddenly multiplication factor changed by keff Increase dn of the number of neutrons n per unit volume dn in the time dt : dn/dt = keff. n / l with l = mean lifetime of the neutrons (time between generations) Solution of the differential equation: n = n 0. exp( keff. t / l) with l / keff = T = Reactor period or e-folding time Neutron flux: n = n 0. exp(t/T) without delayed neutrons

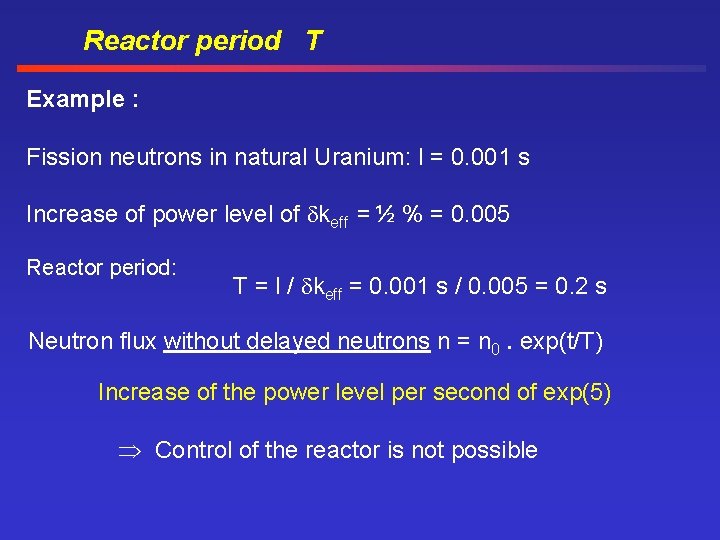
Reactor period T Example : Fission neutrons in natural Uranium: l = 0. 001 s Increase of power level of keff = ½ % = 0. 005 Reactor period: T = l / keff = 0. 001 s / 0. 005 = 0. 2 s Neutron flux without delayed neutrons n = n 0. exp(t/T) Increase of the power level per second of exp(5) Control of the reactor is not possible

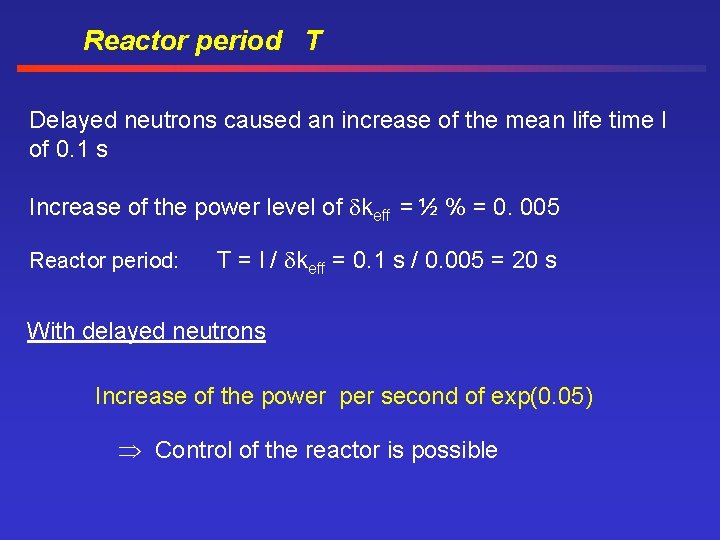
Reactor period T Delayed neutrons caused an increase of the mean life time l of 0. 1 s Increase of the power level of keff = ½ % = 0. 005 Reactor period: T = l / keff = 0. 1 s / 0. 005 = 20 s With delayed neutrons Increase of the power per second of exp(0. 05) Control of the reactor is possible

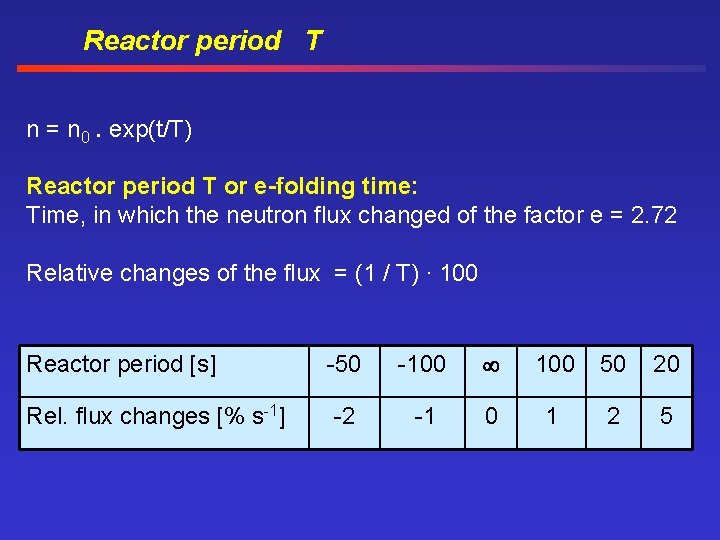
Reactor period T n = n 0. exp(t/T) Reactor period T or e-folding time: Time, in which the neutron flux changed of the factor e = 2. 72 Relative changes of the flux = (1 / T). 100 Reactor period [s] -50 -100 50 20 Rel. flux changes [% s-1] -2 -1 0 1 2 5

Inhour equation The „inhour equation“ gives the relationship between the reactivity and the reactor period in terms of the delayed neutrons (regarding 6 groups) and the prompt neutron lifetime.

Inhour equation Using a time dependent diffusion equation and taking into account the delayed neutrons, it is possible to derive the following equation ρ = (keff - 1) / keff = l / (T keff) + i / (1 + T/ i ) i with i = number of delayed neutrons of the group i i = life time of the delayed neutrons of the group i System of 7 linear in-homogenous differential equations of first order, the differential equations of the reactor kinetics.

Inhour equation Reactivity: ρ = l / (T keff) + / (1 + T/ ) = mean lifetime of the delayed neutrons = i i Case I: Large positive or negative T T >> , 1 << T, ρ << ρ= /T ρ 1/T

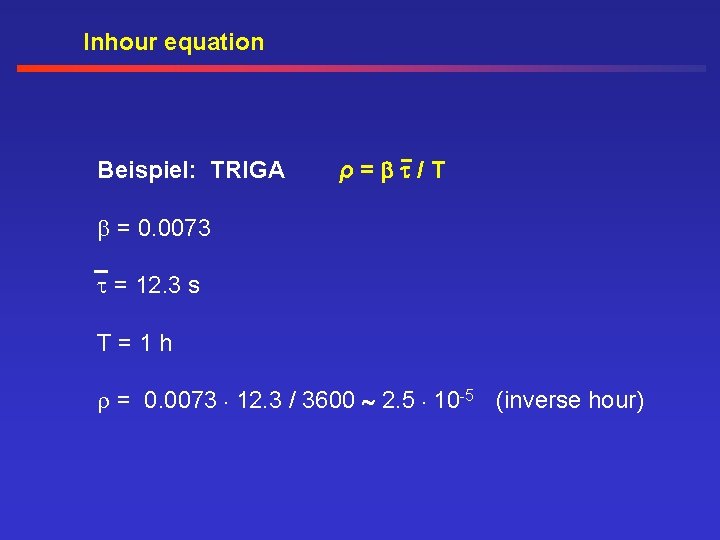
Inhour equation Beispiel: TRIGA ρ= /T = 0. 0073 = 12. 3 s T=1 h = 0. 0073. 12. 3 / 3600 2. 5. 10 -5 (inverse hour)

Inhour equation Reactivity: ρ = l / (T keff) + / (1 + T/ ) Case II: Small positive T, large reactivities ρ 0 < T << , ρ> ρ = l /( keff. T) + T = l / ( keff ( - )) If k is not too far from unit, then T = l / ( - ) If k exceed 1+ß, then the reactor will be critical on prompt neutrons alone. The reactor is prompt critical.
![Inhour equation Für TRIGA 1 dollar 0 0073 1 cent Inhour equation Für TRIGA 1 dollar [$] = = 0. 0073 , 1 cent](https://slidetodoc.com/presentation_image_h/8439376dabcbd64fc75ca4441331dd1c/image-36.jpg)
Inhour equation Für TRIGA 1 dollar [$] = = 0. 0073 , 1 cent = 0. 01 dollar 2 Dollar - Puls ρ = 2 (2 $) = 0. 0146, T = 13. 5 ms

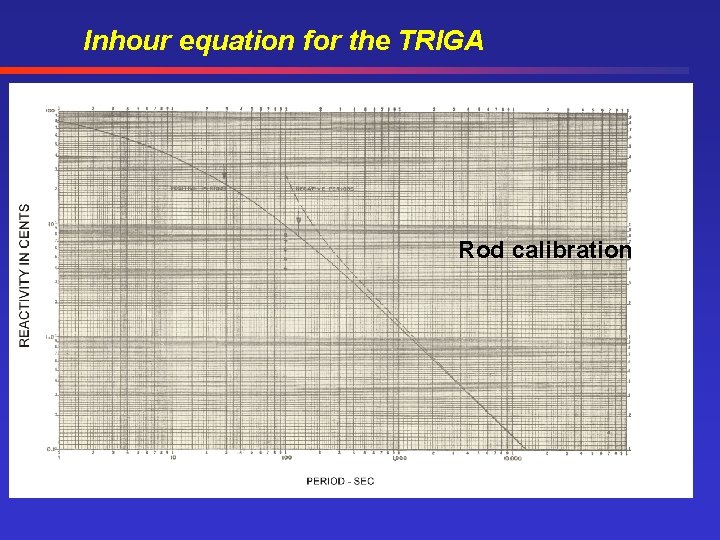
Inhour equation for the TRIGA Rod calibration

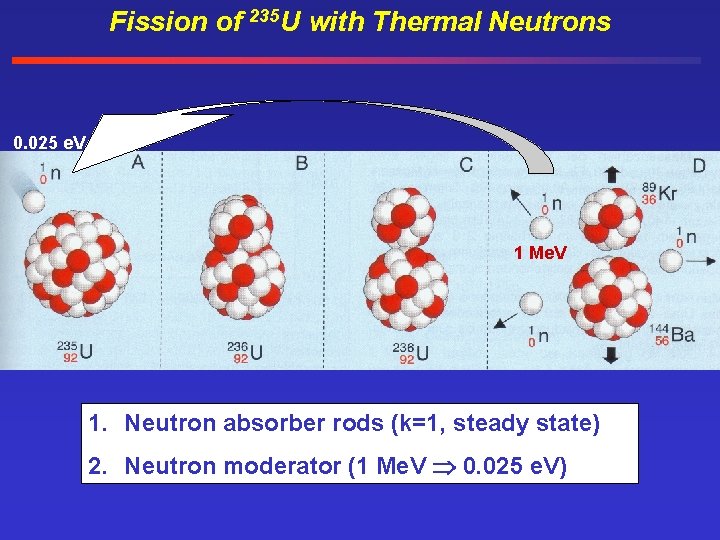
Fission of 235 U with Thermal Neutrons 0. 025 e. V 1 Me. V 1. Neutron absorber rods (k=1, steady state) 2. Neutron moderator (1 Me. V 0. 025 e. V)

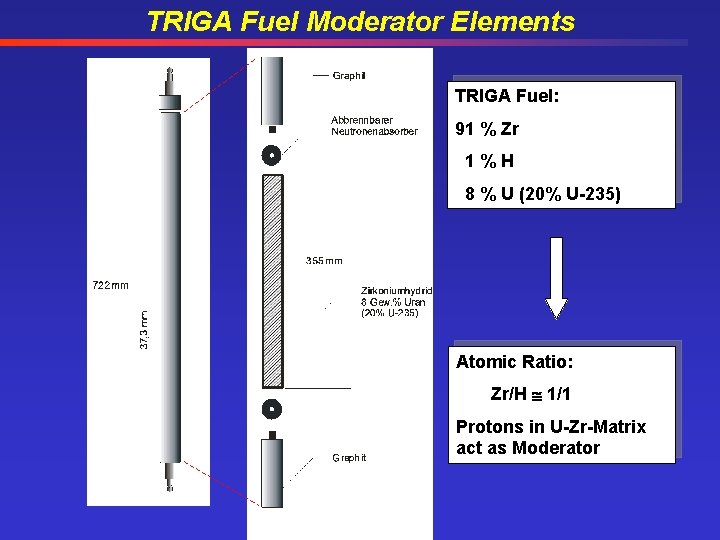
TRIGA Fuel Moderator Elements TRIGA Fuel: 91 % Zr 1%H 8 % U (20% U-235) Atomic Ratio: Zr/H 1/1 Protons in U-Zr-Matrix act as Moderator

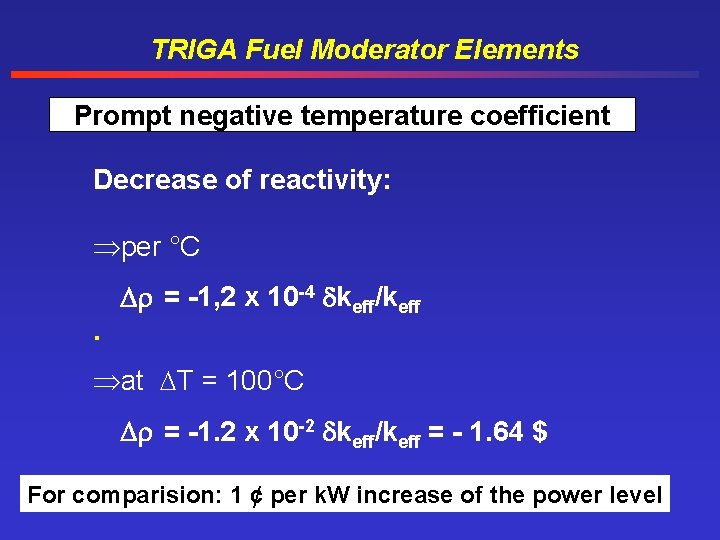
TRIGA Fuel Moderator Elements Prompt negative temperature coefficient Decrease of reactivity: per °C. = -1, 2 x 10 -4 keff/keff at T = 100°C = -1. 2 x 10 -2 keff/keff = - 1. 64 $ For comparision: 1 ¢ per k. W increase of the power level

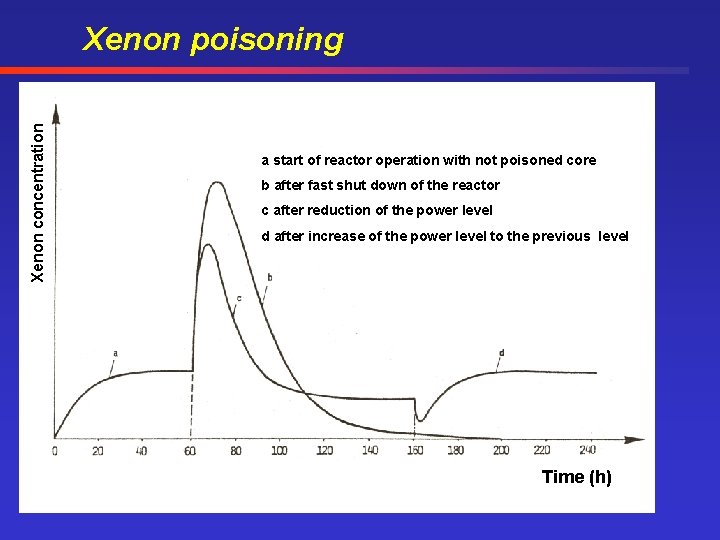
Xenon poisoning Reason for the Xenon poisoning: Decay of I-135 into Xe-135 (large capture cross section for thermal neutrons) • Operation at constant power level: Production of I-135 and Xe • Increase of the power level: increase of I-135 and Xe • Decrease of the power level: Xe concentration increases due to the decay of the I-135 and neutrons will be captured by Xe. With a delay time of the half lifetime of I-135 the capture of neutrons decreases and also the power decreases slowly. • Operation of the reactor after the shut down is not possible when the absorption by Xe is too large. • Operation possible, when the Xe-135 production is negligible (about 20 h)

Xenon concentration Xenon poisoning a start of reactor operation with not poisoned core b after fast shut down of the reactor c after reduction of the power level d after increase of the power level to the previous level Time (h)

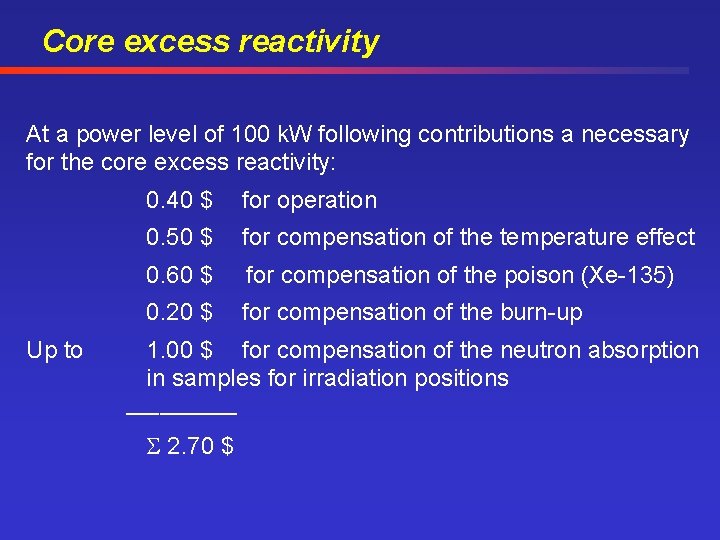
Core excess reactivity At a power level of 100 k. W following contributions a necessary for the core excess reactivity: Up to 0. 40 $ for operation 0. 50 $ for compensation of the temperature effect 0. 60 $ for compensation of the poison (Xe-135) 0. 20 $ for compensation of the burn-up 1. 00 $ for compensation of the neutron absorption in samples for irradiation positions _____ 2. 70 $

Summary 1. Nuclear fission 2. Reactivity 3. Ideal (infinite) and real (finite) reactor 4. Inhour equation 5. TRIGA fuel (moderation, prompt negative temperature coefficient, inherent safe reactor) 6. Xenon poisoning

More information to the operation of the TRIGA Mainz 1. Structure 2. Instrumentation 3. Cooling- and purification circuits 4. Radiation protection 5. Safety 6. Checks (internal – external) 7. Special incidents 8. Documentation 9. Organization