The role of allogeneic transplantation in peripheral Tcell







- Slides: 29

The role of allogeneic transplantation in peripheral T-cell lymphomas Paolo Corradini, M. D. Dept. of Hematology and Bone Marrow Transplantation – University of Milano, Istituto Nazionale per lo Studio e la Cura dei Tumori, Milano, Italy

A very difficult task ! La Palud sur Verdon, France

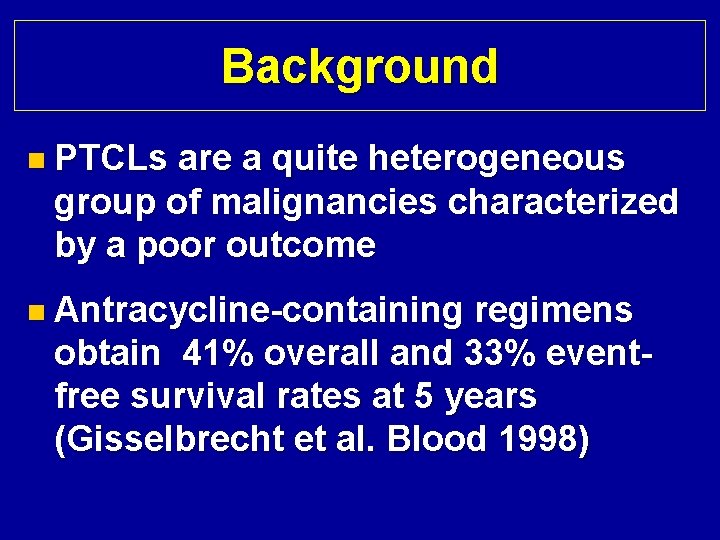
Background n PTCLs are a quite heterogeneous group of malignancies characterized by a poor outcome n Antracycline-containing regimens obtain 41% overall and 33% eventfree survival rates at 5 years (Gisselbrecht et al. Blood 1998)

Question # 1: Do we need to intensify therapy in PTCL ? Question #2: auto or allo-SCT ?

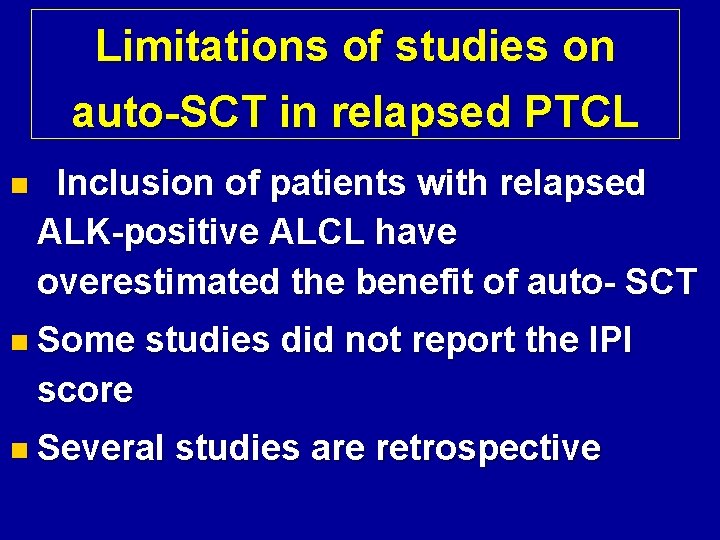
Limitations of studies on auto-SCT in relapsed PTCL n Inclusion of patients with relapsed ALK-positive ALCL have overestimated the benefit of auto- SCT n Some studies did not report the IPI score n Several studies are retrospective

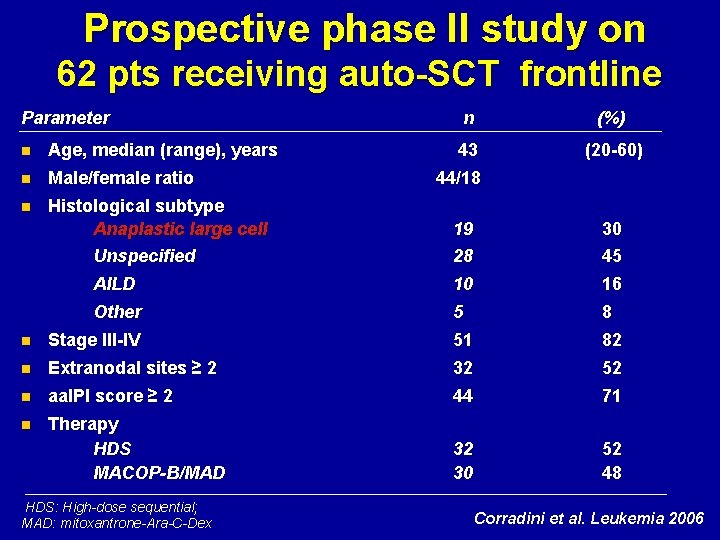
Prospective phase II study on 62 pts receiving auto-SCT frontline Parameter n Age, median (range), years n Male/female ratio n Histological subtype Anaplastic large cell Unspecified n (%) 43 (20 -60) 44/18 19 28 30 45 AILD 10 16 Other 5 8 n Stage III-IV 51 82 n Extranodal sites ≥ 2 32 52 n aa. IPI score ≥ 2 44 71 n Therapy HDS MACOP-B/MAD 32 30 52 48 HDS: High-dose sequential; MAD: mitoxantrone-Ara-C-Dex Corradini et al. Leukemia 2006

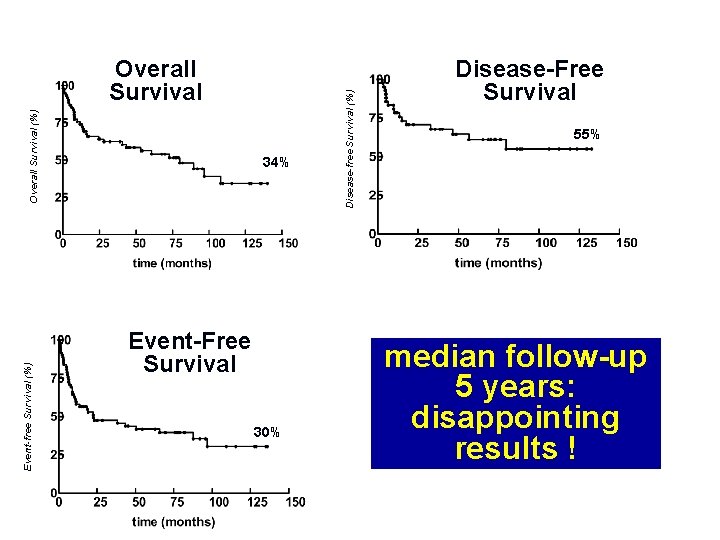
Overall Survival (%) Overall Survival 34% Disease-free Survival (%) B Disease-Free Survival 55% Event-free Survival (%) C Event-Free Survival 30% median follow-up 5 years: disappointing results !

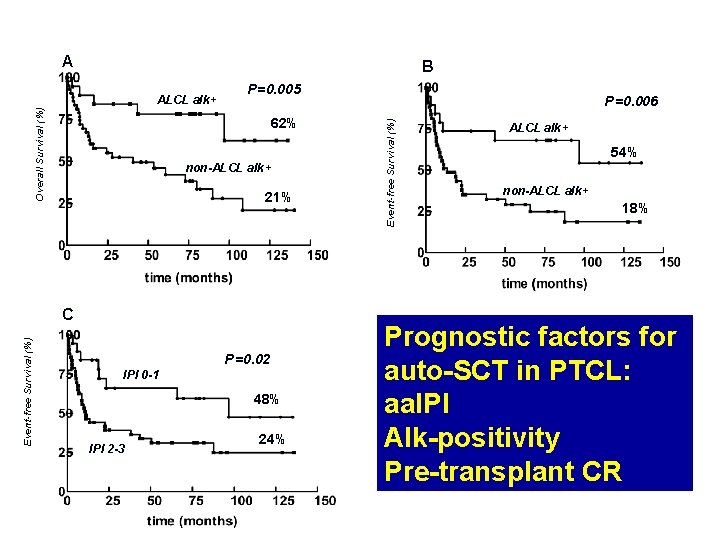
Figure 2 A B P=0. 005 62% non-ALCL alk+ 21% Event-free Survival (%) C P=0. 02 IPI 0 -1 48% IPI 2 -3 24% P=0. 006 Event-free Survival (%) Overall Survival (%) ALCL alk+ 54% non-ALCL alk+ 18% Prognostic factors for auto-SCT in PTCL: aa. IPI Alk-positivity Pre-transplant CR

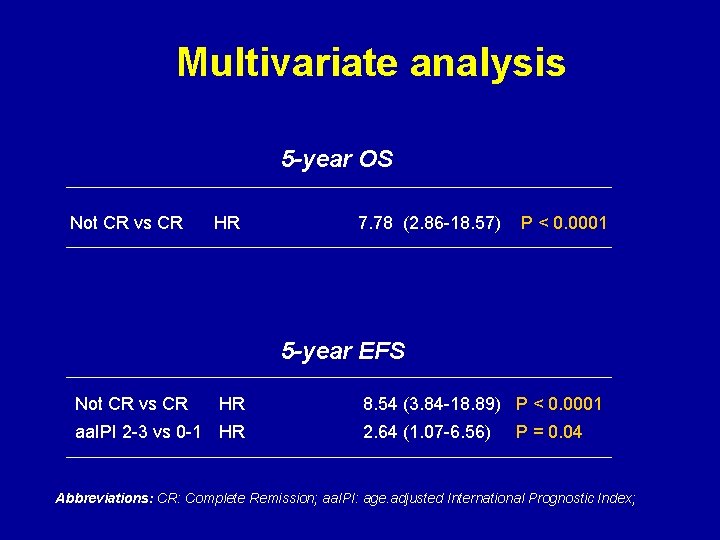
Multivariate analysis 5 -year OS Not CR vs CR HR 7. 78 (2. 86 -18. 57) P < 0. 0001 5 -year EFS Not CR vs CR HR aa. IPI 2 -3 vs 0 -1 HR 8. 54 (3. 84 -18. 89) P < 0. 0001 2. 64 (1. 07 -6. 56) P = 0. 04 Abbreviations: CR: Complete Remission; aa. IPI: age. adjusted International Prognostic Index;

What about the Graft-versus. Lymphoma effect in PTCL ? In the last 20 years very few allotransplants were performed with some long-term responses, but with a particularly high non-relapse mortality

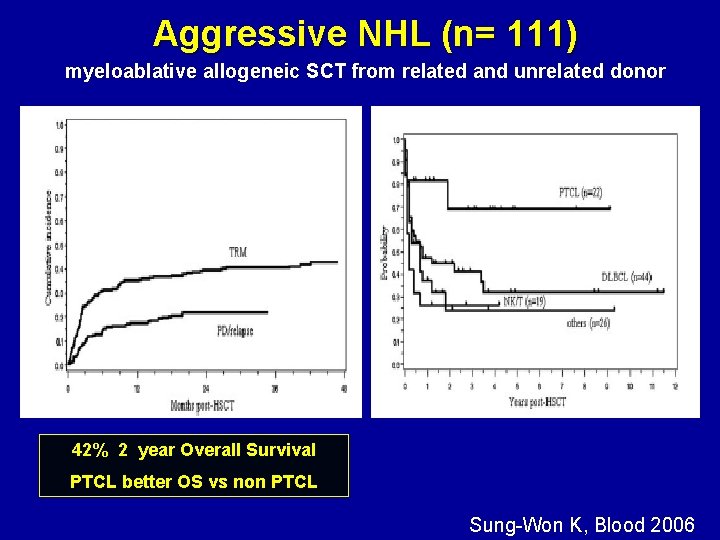
Aggressive NHL (n= 111) myeloablative allogeneic SCT from related and unrelated donor 42% 2 year Overall Survival PTCL better OS vs non PTCL Sung-Won K, Blood 2006

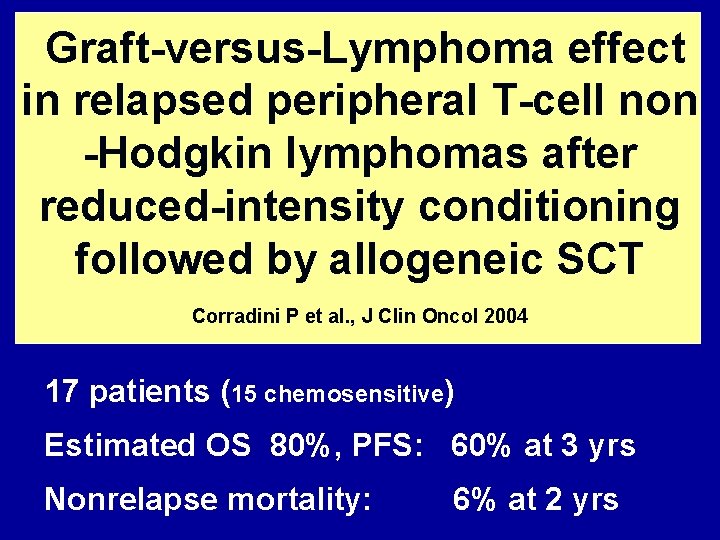
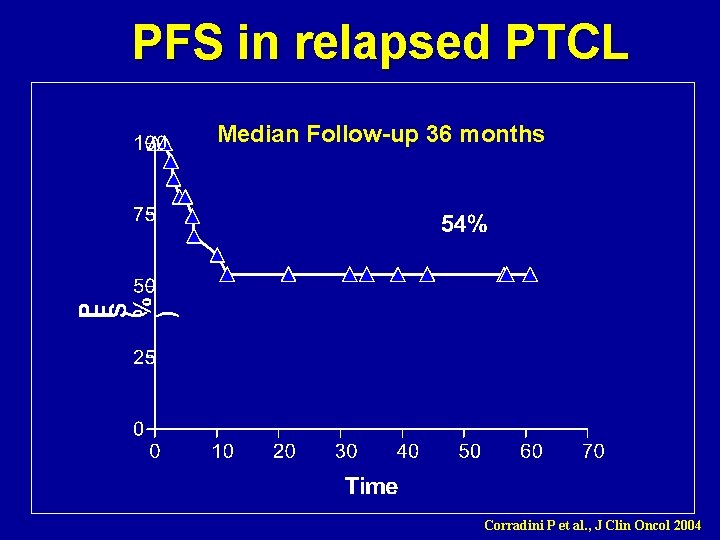
Graft-versus-Lymphoma effect in relapsed peripheral T-cell non -Hodgkin lymphomas after reduced-intensity conditioning followed by allogeneic SCT Corradini P et al. , J Clin Oncol 2004 17 patients (15 chemosensitive) Estimated OS 80%, PFS: 60% at 3 yrs Nonrelapse mortality: 6% at 2 yrs

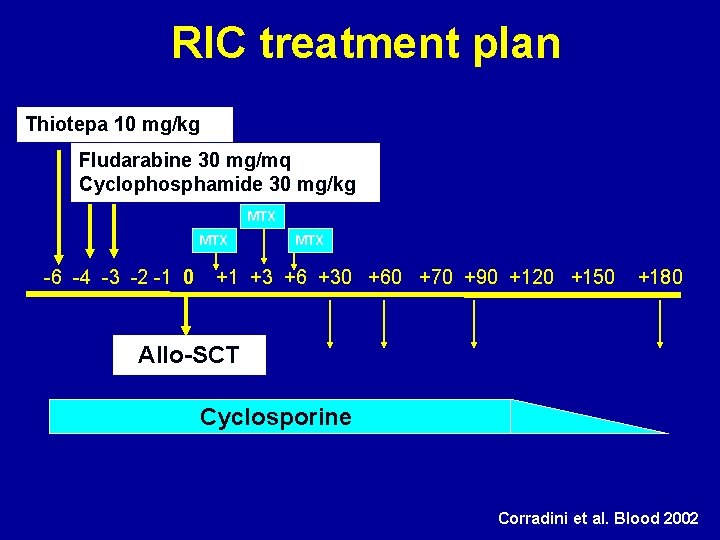
RIC treatment plan Thiotepa 10 mg/kg Fludarabine 30 mg/mq Cyclophosphamide 30 mg/kg MTX -6 -4 -3 -2 -1 0 MTX +1 +3 +6 +30 +60 +70 +90 +120 +150 +180 Allo-SCT Cyclosporine Corradini et al. Blood 2002

PFS in relapsed PTCL Median Follow-up 36 months Corradini P et al. , J Clin Oncol 2004

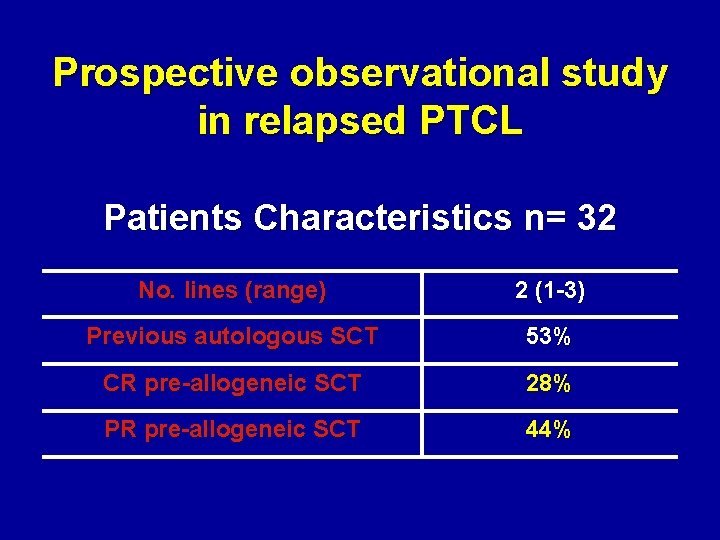
Prospective observational study in relapsed PTCL Patients Characteristics n= 32 No. lines (range) 2 (1 -3) Previous autologous SCT 53% CR pre-allogeneic SCT 28% PR pre-allogeneic SCT 44%

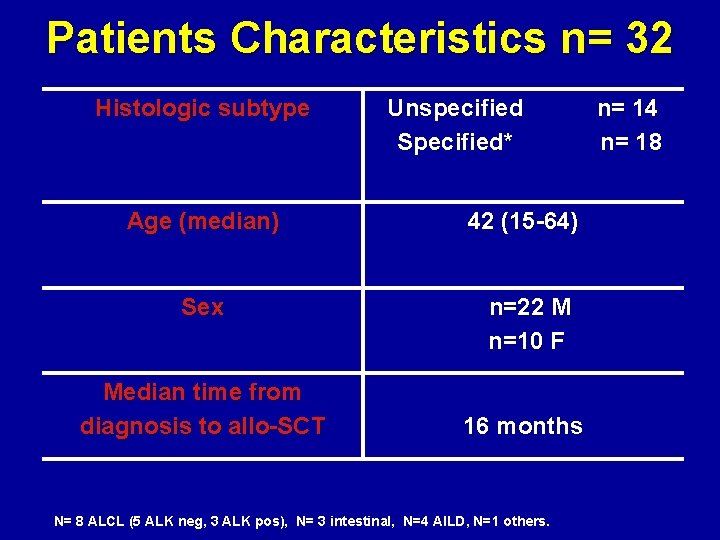
Patients Characteristics n= 32 Histologic subtype Unspecified Specified* Age (median) 42 (15 -64) Sex n=22 M n=10 F Median time from diagnosis to allo-SCT 16 months N= 8 ALCL (5 ALK neg, 3 ALK pos), N= 3 intestinal, N=4 AILD, N=1 others. n= 14 n= 18

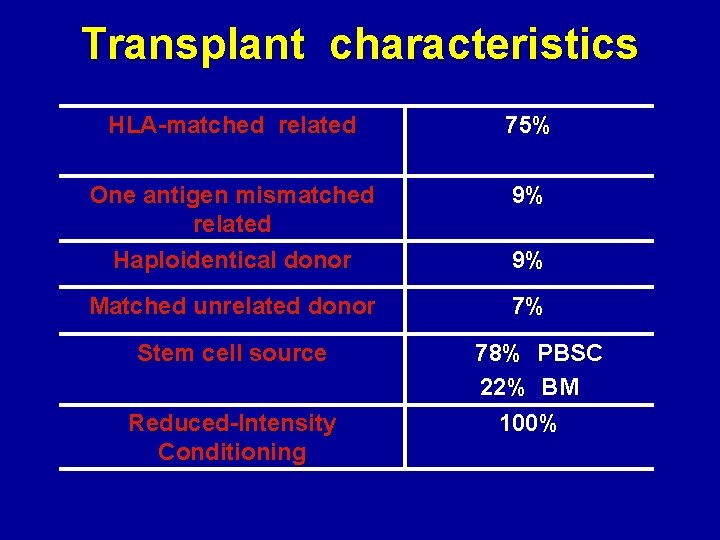
Transplant characteristics HLA-matched related 75% One antigen mismatched related Haploidentical donor 9% Matched unrelated donor 7% Stem cell source Reduced-Intensity Conditioning 9% 78% PBSC 22% BM 100%

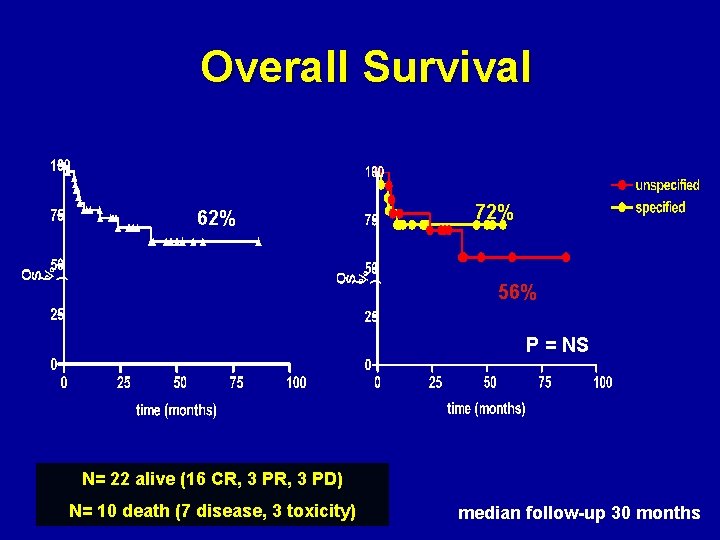
Overall Survival 62% 72% 56% P = NS N= 22 alive (16 CR, 3 PD) N= 10 death (7 disease, 3 toxicity) median follow-up 30 months

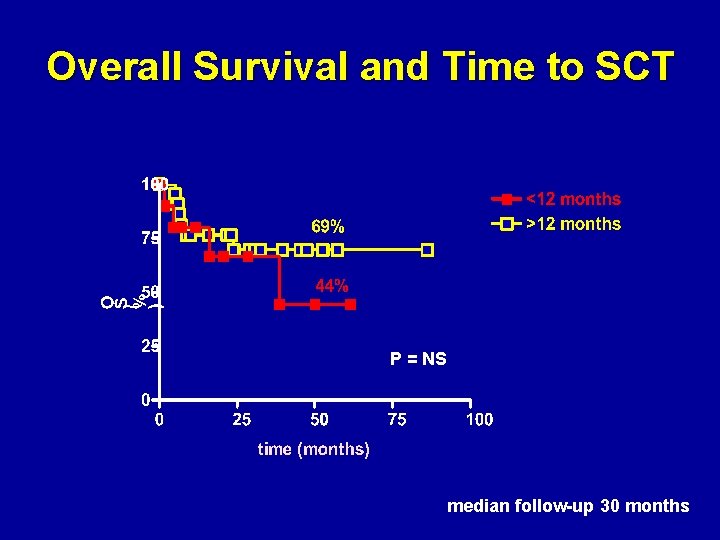
Overall Survival and Time to SCT P = NS median follow-up 30 months

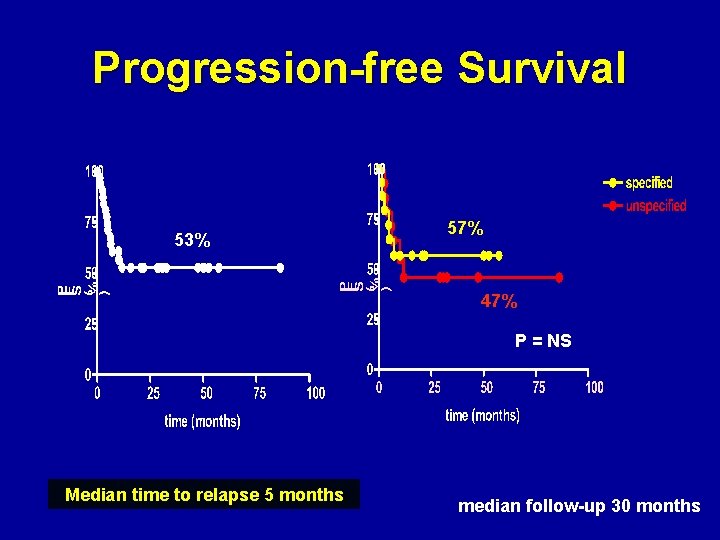
Progression-free Survival 53% 57% 47% P = NS Median time to relapse 5 months median follow-up 30 months

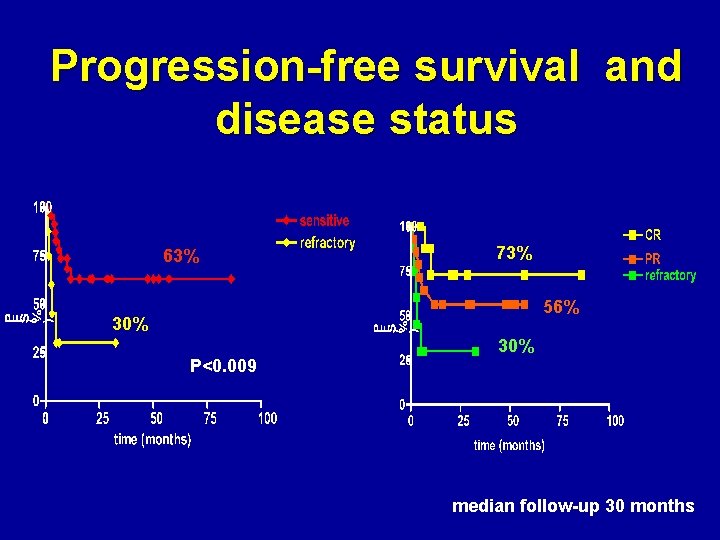
Progression-free survival and disease status 63% 73% 56% 30% P<0. 009 30% median follow-up 30 months

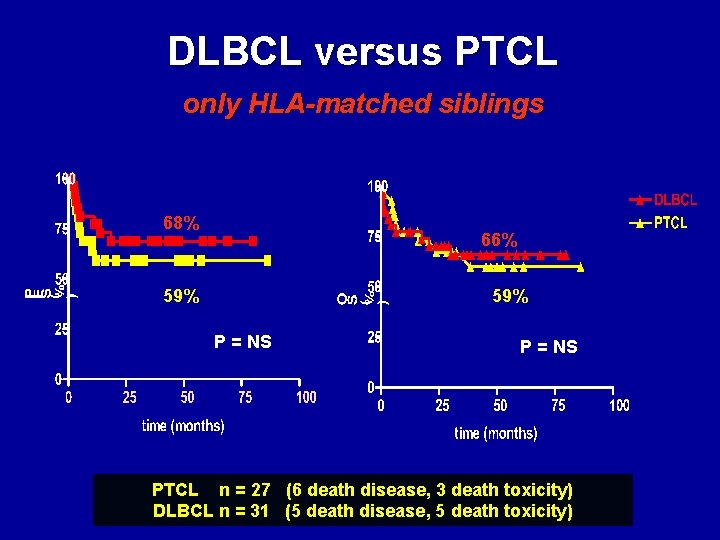
DLBCL versus PTCL only HLA-matched siblings 68% 66% 59% P = NS PTCL n = 27 (6 death disease, 3 death toxicity) DLBCL n = 31 (5 death disease, 5 death toxicity)

Conclusions n RIC allo-SCT has decreased NRM n Allo-SCT has showed promising results for disease control in relapsed patients n Allo-SCT frontline ? n New agents are urgently required !

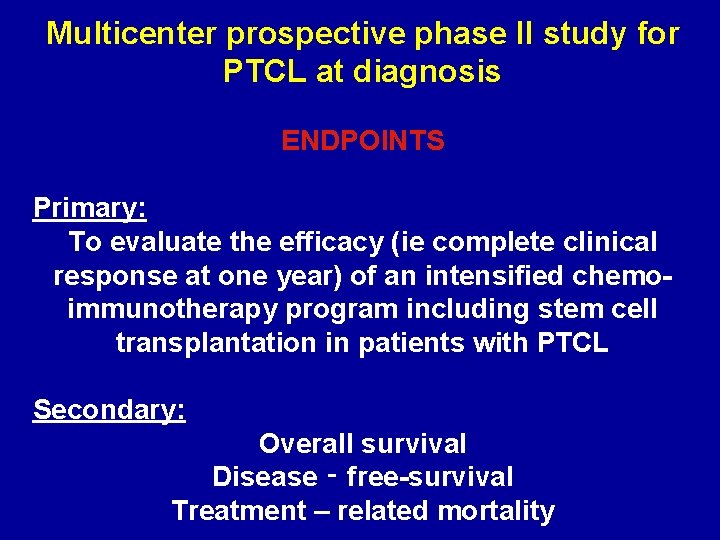
Multicenter prospective phase II study for PTCL at diagnosis ENDPOINTS Primary: To evaluate the efficacy (ie complete clinical response at one year) of an intensified chemoimmunotherapy program including stem cell transplantation in patients with PTCL Secondary: Overall survival Disease ‑ free-survival Treatment – related mortality

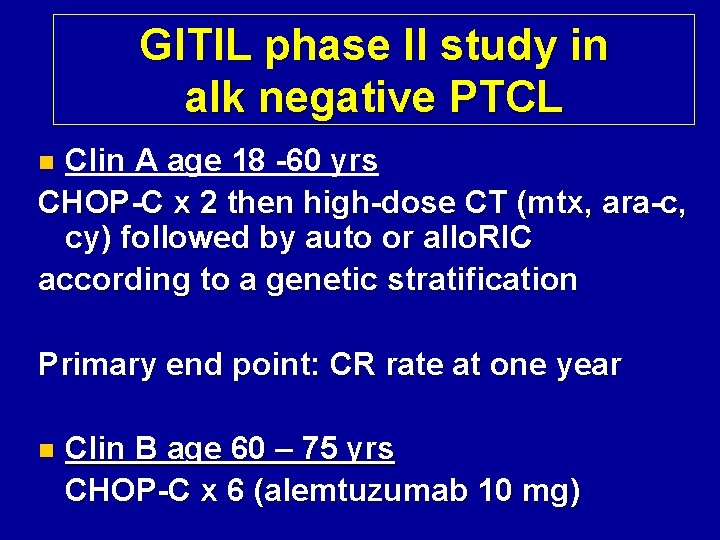
GITIL phase II study in alk negative PTCL Clin A age 18 -60 yrs CHOP-C x 2 then high-dose CT (mtx, ara-c, cy) followed by auto or allo. RIC according to a genetic stratification n Primary end point: CR rate at one year n Clin B age 60 – 75 yrs CHOP-C x 6 (alemtuzumab 10 mg)

Inclusion criteria: • Age ≥ 18 60 yrs (clin A); >60 <75 yrs (clin B) • Histologically proven diagnosis of PTCL, including the following categories: PTCL-U, AILD -T, ALKneg ALCL (ALK-negative anaplastic large cell lymphoma), intestinal T – NHL • Advanced stage disease (stage II-IV) or stage I and aa. IPI score ≥ 2 • CD 52 expression on neoplastic cells

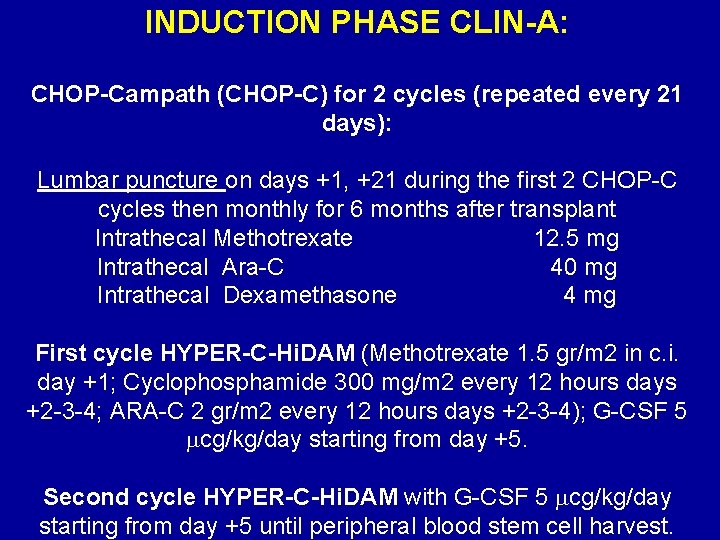
INDUCTION PHASE CLIN-A: CHOP-Campath (CHOP-C) for 2 cycles (repeated every 21 days): Lumbar puncture on days +1, +21 during the first 2 CHOP-C cycles then monthly for 6 months after transplant Intrathecal Methotrexate 12. 5 mg Intrathecal Ara-C 40 mg Intrathecal Dexamethasone 4 mg First cycle HYPER-C-Hi. DAM (Methotrexate 1. 5 gr/m 2 in c. i. day +1; Cyclophosphamide 300 mg/m 2 every 12 hours days +2 -3 -4; ARA-C 2 gr/m 2 every 12 hours days +2 -3 -4); G-CSF 5 cg/kg/day starting from day +5. Second cycle HYPER-C-Hi. DAM with G-CSF 5 cg/kg/day starting from day +5 until peripheral blood stem cell harvest.

Patients in PR or CR without a HLA-identical sibling or unrelated donor: autologous transplantation: BEAM regimen followed by reinfusion of PBSC on day 0 (>4 x 10 e 6 CD 34+/kg). Patients in PR o CR with a HLA-identical donor: allogeneic transplantation: Conditioning regimen: Thiotepa 15 mg/kg (day – 6) for patient < 45 years and 10 mg/ kg for patient ≥ 45 years; cyclophosphamide 30 mg/kg (days – 4 and – 3); fludarabine 30 mg/m 2 (days – 4 and – 3) followed by allo. SCT from a HLA-identical (or one antigen mismatched) sibling or from an allele matched unrelated donor on day 0 (5– 8 x 10 e 6 CD 34+ cell/kg). ATG will be part of the conditioning for mismatched siblings and unrelated donors. GVHD prophylaxis: CSA, adjusted to 200 -300 ng/ml blood levels, and short course methotrexate (10 mg/m 2 day +1, 8 mg/m 2 day +3 and +6). In patient in CR after transplantation CSA is administered at full dose through day +100 and, if GVHD did not occur, the dose is tapered by 10% every 10 days thereafter.

Aknowledgments Dept. of Hematology Istituto Nazionale dei Tumori University of Milano A. Dodero, R. Milani, F Zallio V. Montefusco, C. Carniti Dept. of Medical Oncology Istituto Nazionale Tumori University of Milano A. M. Gianni, P. Matteucci Dept. of Hematology University of Torino C. Tarella, M. Boccadoro Dept. of Hematology Ospedali Riuniti, Bergamo A. Rambaldi T. Barbui B. Dept Hematology C. Bolzano Hospital D. S Cortelazzo Rijukanfossen Norway GITIL and GITMO study groups
 Oncogens
Oncogens Allogeneic stem cell transplant
Allogeneic stem cell transplant Leukemoid reaction
Leukemoid reaction How does a kidney transplant work
How does a kidney transplant work Law of transplantation
Law of transplantation Stem cell phuket
Stem cell phuket Patrick evrard mont godinne
Patrick evrard mont godinne Cultural transplantation examples
Cultural transplantation examples Bone marrow transplantation sri lanka
Bone marrow transplantation sri lanka Statuses and their related roles determine
Statuses and their related roles determine Azure worker role
Azure worker role Ambiguitätstoleranz krappmann
Ambiguitätstoleranz krappmann Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Lời thề hippocrates
Lời thề hippocrates Bổ thể
Bổ thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi điện thế nghỉ
điện thế nghỉ Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Gấu đi như thế nào
Gấu đi như thế nào Các số nguyên tố
Các số nguyên tố Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Tia chieu sa te
Tia chieu sa te Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Một số thể thơ truyền thống
Một số thể thơ truyền thống