The Origin and Nature of Light The Origin


















- Slides: 42

The Origin and Nature of Light

The Origin and Nature of Light • Celebration of Knowledge #2 (aka Exam #2) is Thursday March 8 th in N 210 • Tailgate Party (aka exam review) is Wednesday March 7 th in N 210 from 4 -6 pm • HW #5 – Handed out in class Feb 27 th on the topic of Luminosity Area and Temperature, and Due INCLASS Tuesday March 6 th

• HW#6 – Masteringastronomy online homework on The Origin and Nature of Light Properties of Light and Matter. Available March 1 st , Due March 8 th by 10 am.

What can we learn by analyzing starlight? • A star’s temperature

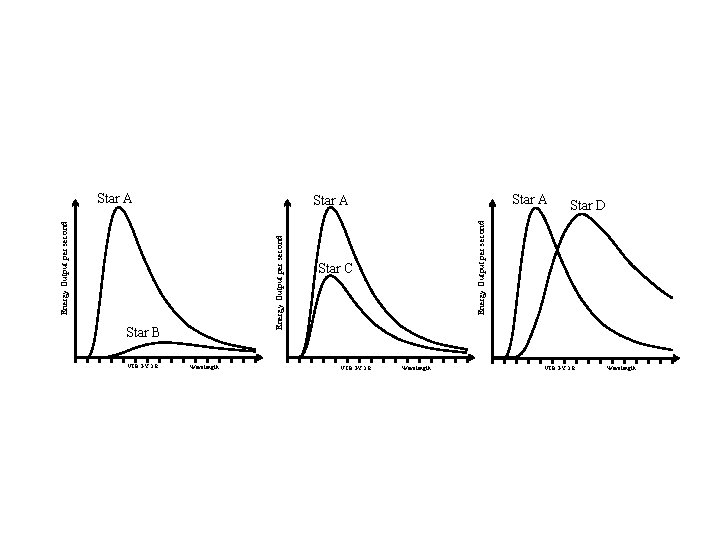
Find the hottest star(s), how do you know ?

Star A Wavelength Star C VIBGYOR Star D Energy Output per second Star B VIBGYOR Star A Wavelength VIBGYOR Wavelength

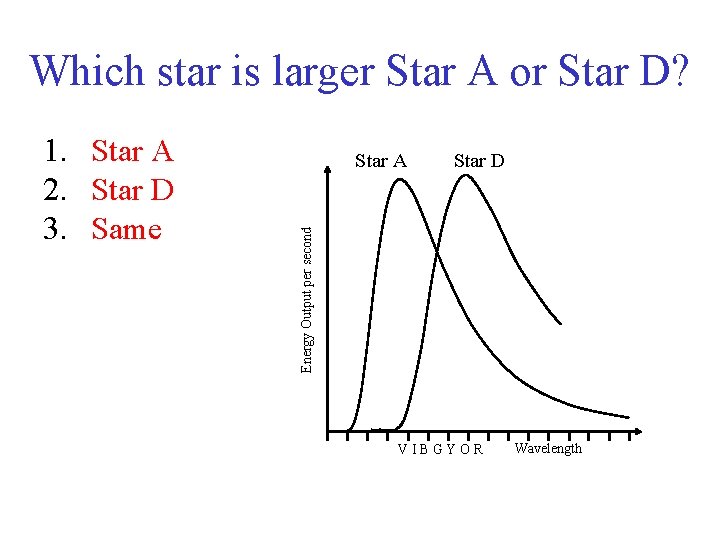
Which star is larger Star A or Star D? Star A Star D Energy Output per second 1. Star A 2. Star D 3. Same VIBGYOR Wavelength

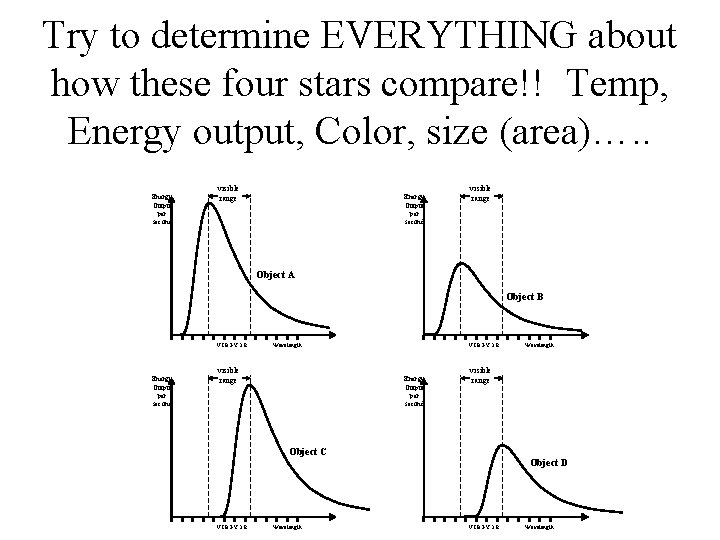
Try to determine EVERYTHING about how these four stars compare!! Temp, Energy output, Color, size (area)…. . Energy Output per second visible range Object A Object B VIBGYOR Energy Output per second Wavelength visible range VIBGYOR Energy Output per second visible range Object C VIBGYOR Wavelength Object D VIBGYOR Wavelength

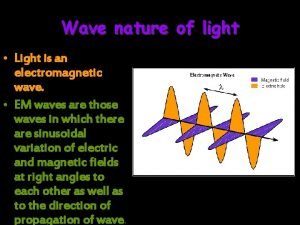
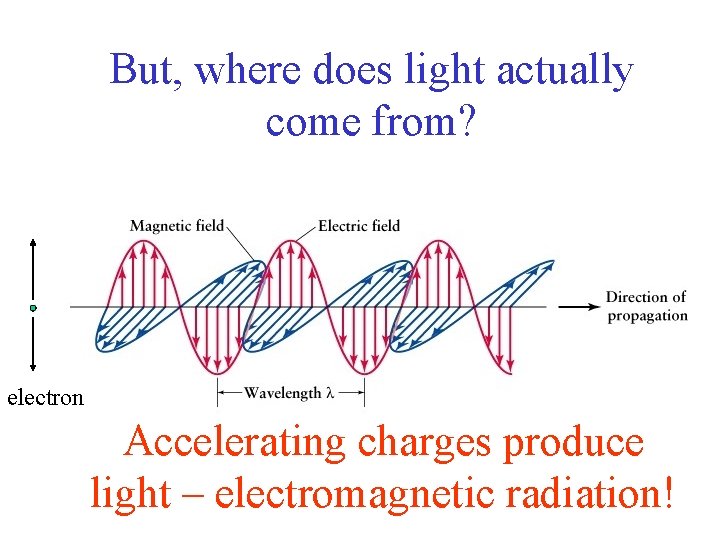
But, where does light actually come from? electron Accelerating charges produce light – electromagnetic radiation!

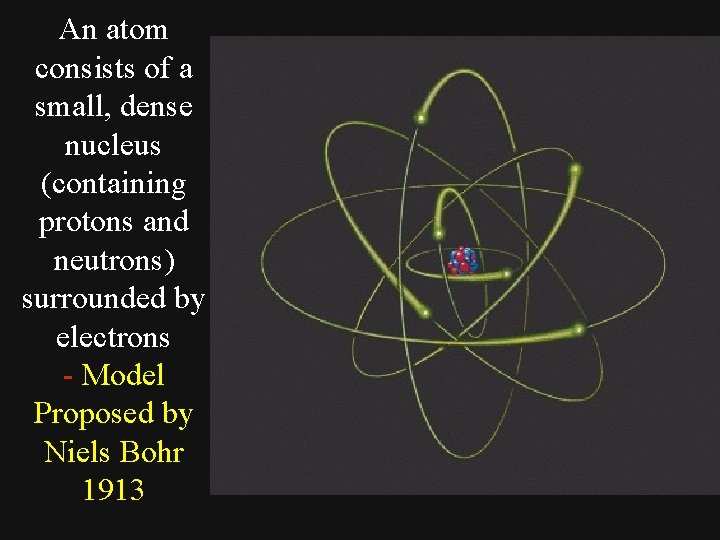
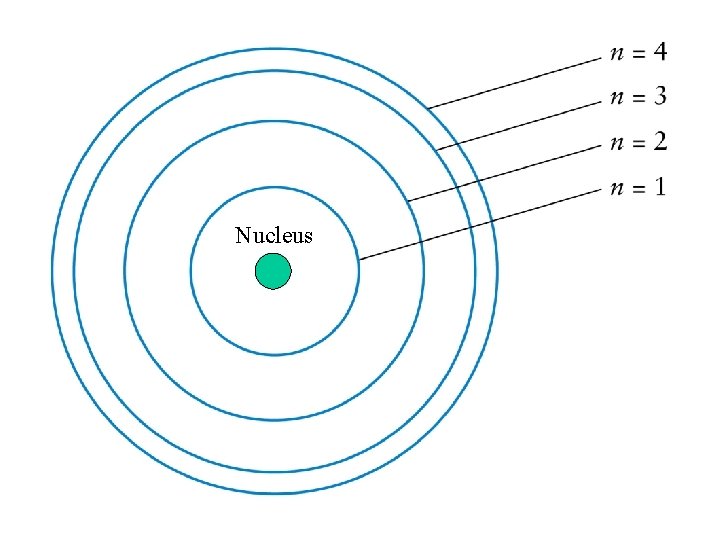
An atom consists of a small, dense nucleus (containing protons and neutrons) surrounded by electrons - Model Proposed by Niels Bohr 1913

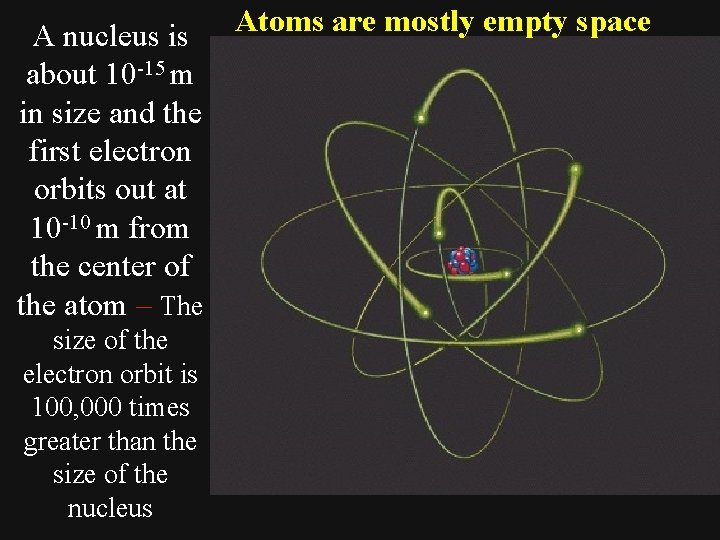
A nucleus is about 10 -15 m in size and the first electron orbits out at 10 -10 m from the center of the atom – The size of the electron orbit is 100, 000 times greater than the size of the nucleus Atoms are mostly empty space

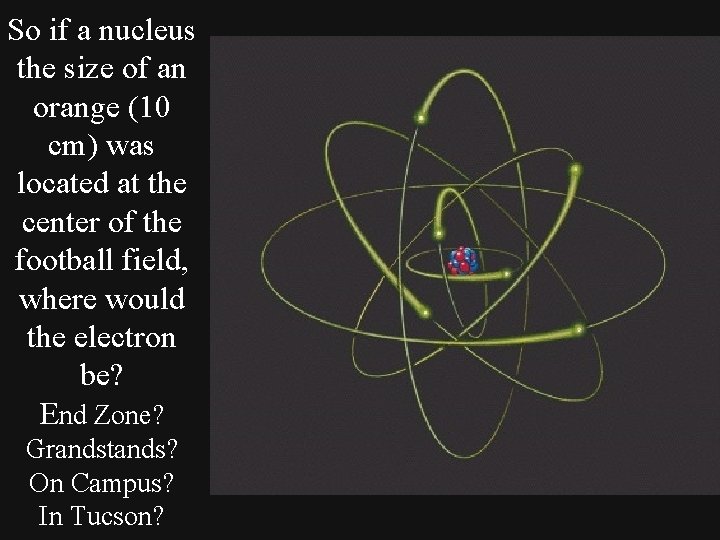
So if a nucleus the size of an orange (10 cm) was located at the center of the football field, where would the electron be? End Zone? Grandstands? On Campus? In Tucson?

If the electron’s orbit is 100, 000 times bigger than the nucleus then the electron would be 10, 000 m or 6. 21 miles away from the center of the Football Field! Still in Tucson, up in the foothills shopping at La Encantada!!

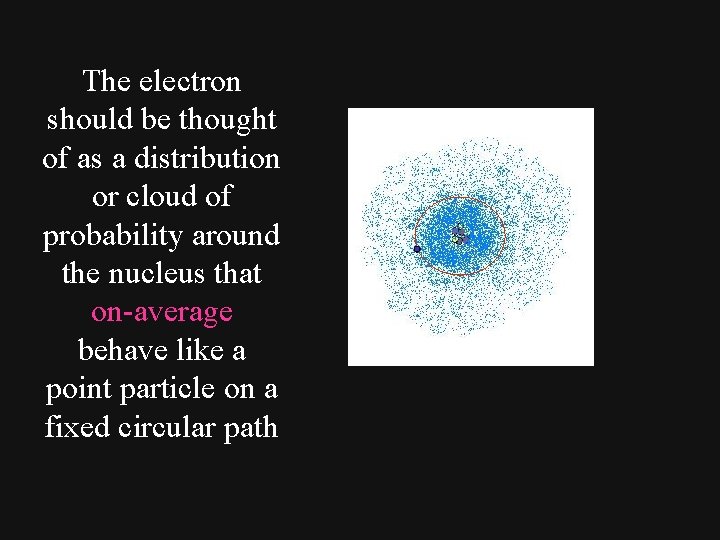
The electron should be thought of as a distribution or cloud of probability around the nucleus that on-average behave like a point particle on a fixed circular path

Nucleus

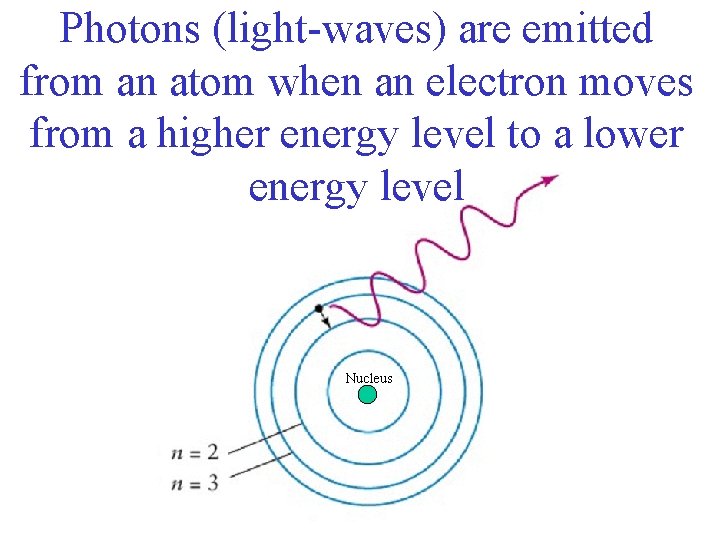
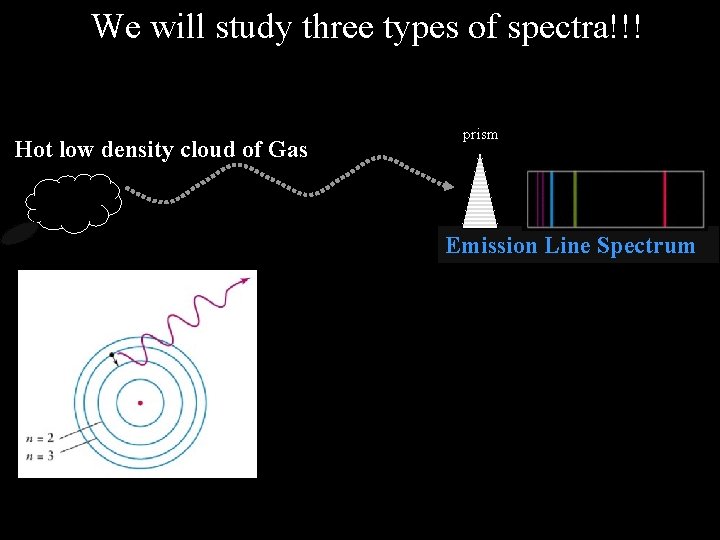
Photons (light-waves) are emitted from an atom when an electron moves from a higher energy level to a lower energy level Nucleus

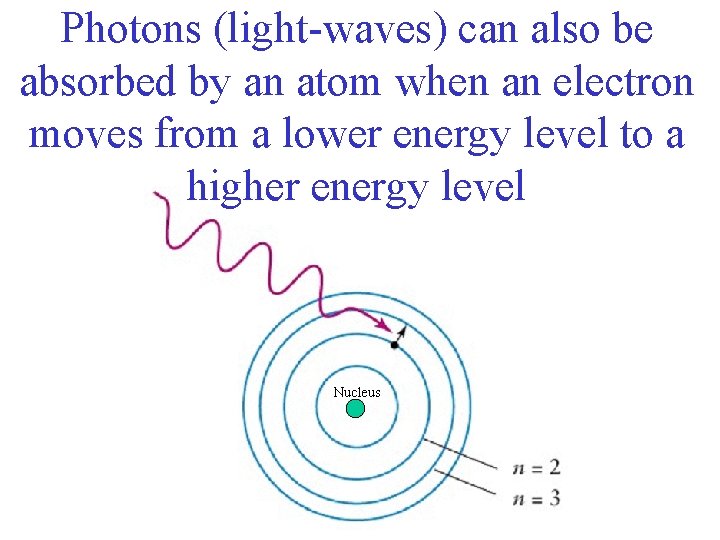
Photons (light-waves) can also be absorbed by an atom when an electron moves from a lower energy level to a higher energy level Nucleus



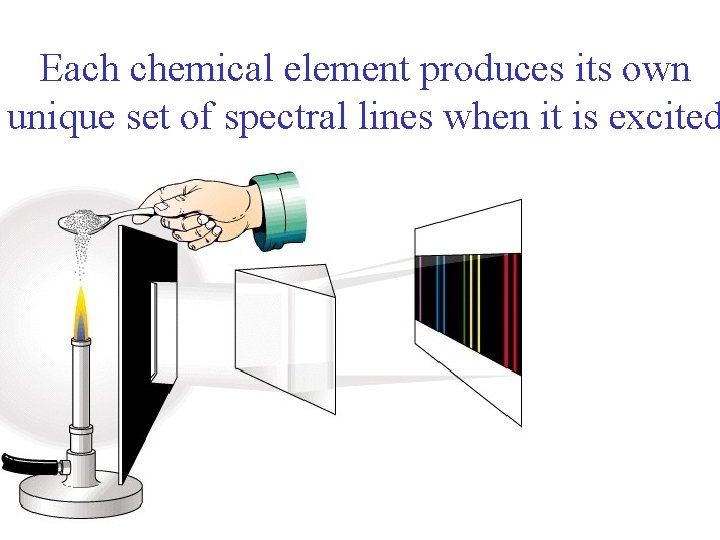
Each chemical element produces its own unique set of spectral lines when it is excited

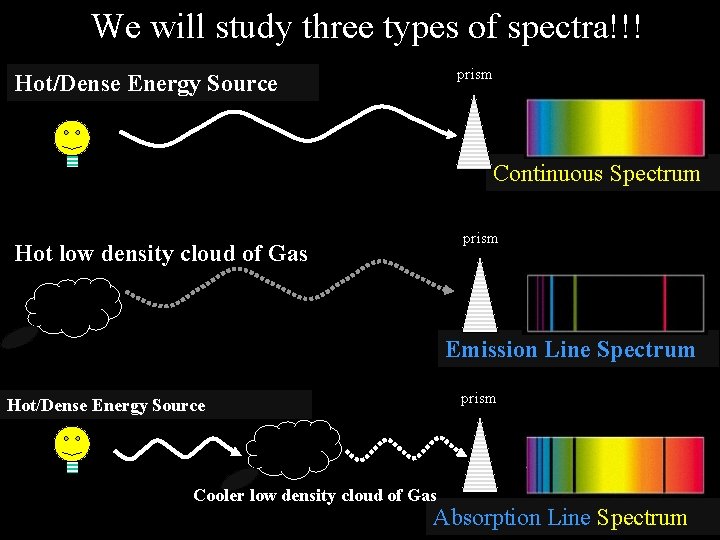
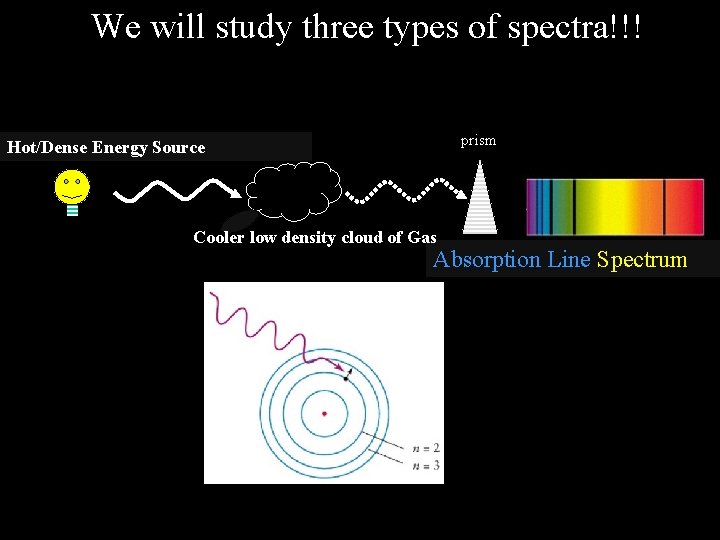
We will study three types of spectra!!! prism Hot/Dense Energy Source Continuous Spectrum prism Hot low density cloud of Gas Emission Line Spectrum prism Hot/Dense Energy Source Cooler low density cloud of Gas Absorption Line Spectrum

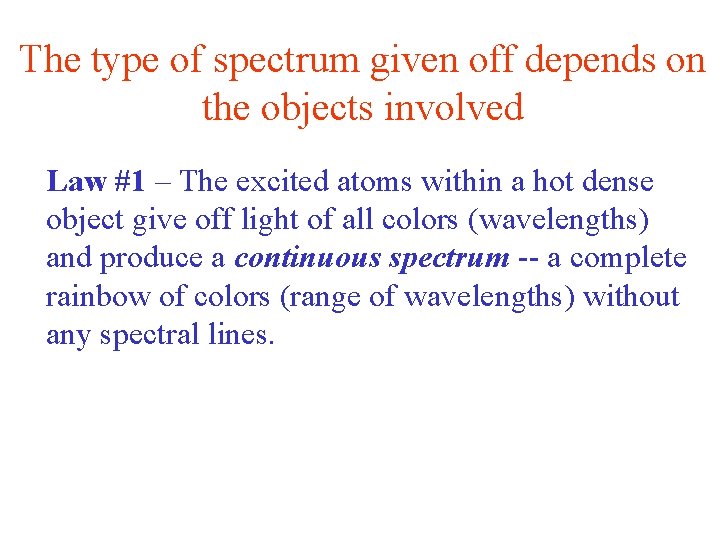
The type of spectrum given off depends on the objects involved Law #1 – The excited atoms within a hot dense object give off light of all colors (wavelengths) and produce a continuous spectrum -- a complete rainbow of colors (range of wavelengths) without any spectral lines.

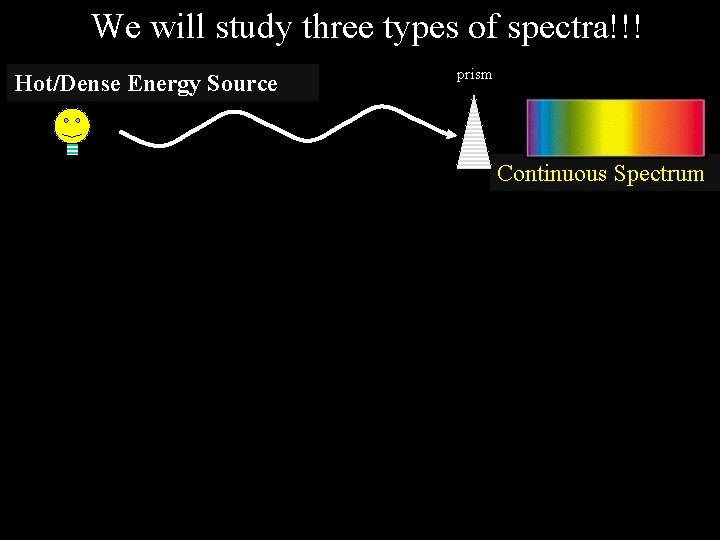
We will study three types of spectra!!! Hot/Dense Energy Source prism Continuous Spectrum

The type of spectrum given off depends on the objects involved Law #2 – The excited atoms within a hot, cloud of gas give off only particular colors (wavelengths) of light and produce an emission line spectrum - a series of bright spectral lines against a dark background.

We will study three types of spectra!!! Hot low density cloud of Gas prism Emission Line Spectrum

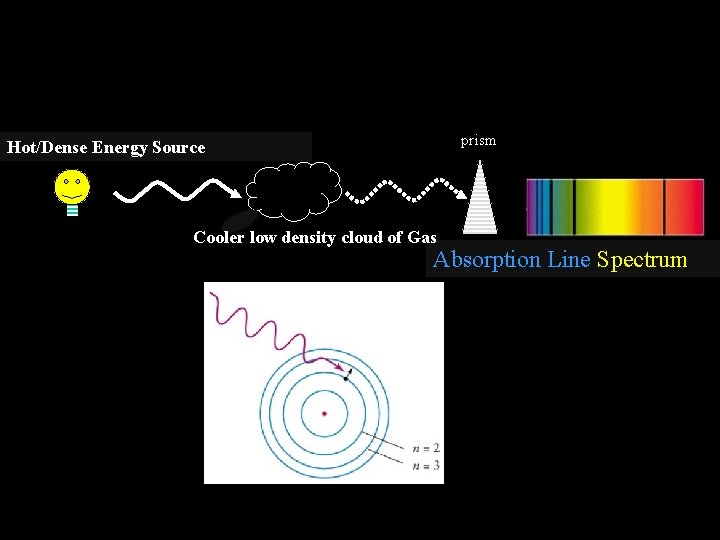
The type of spectrum given off depends on the objects involved Law #3 – When the light from a hot dense object passes through a cool cloud of gas, the atoms within the cloud can absorb particular colors (wavelengths) of light and produce a absorption line spectrum - a series of dark spectral lines among the colors of the rainbow.

We will study three types of spectra!!! prism Hot/Dense Energy Source Cooler low density cloud of Gas Absorption Line Spectrum

Tutorial: Types of Spectra – p. 41 • Work with a partner! • Read the instructions and questions carefully. • Discuss the concepts and your answers with one another. Take time to understand it now!!!! • Come to a consensus answer you both agree on. • If you get stuck or are not sure of your answer, ask another group.

Tutorial: Light and Atoms – LT Handout • Work with a partner! • Read the instructions and questions carefully. • Discuss the concepts and your answers with one another. Take time to understand it now!!!! • Come to a consensus answer you both agree on. • If you get stuck or are not sure of your answer, ask another group.

Imagine that you observe the Sun using a telescope in an orbit high above Earth’s atmosphere. Which of the following spectra would you observe by analyzing the sunlight? 1. 2. 3. 4. dark line absorption spectrum bright line emission spectrum continuous spectrum None of the above

If an electron in an atom moves from an orbit with an energy of 5 to an orbit with an energy of 10, A. a photon of energy 5 is emitted B. a photon of energy 15 is emitted. C. a photon of energy 5 is absorbed. D. a photon of energy 15 is absorbed. E. None of the above

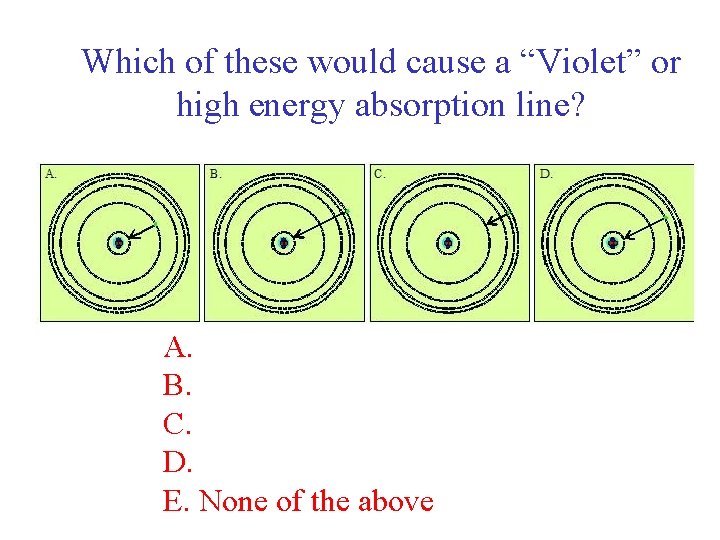
Which of these would cause a “Violet” or high energy absorption line? A. B. C. D. E. None of the above

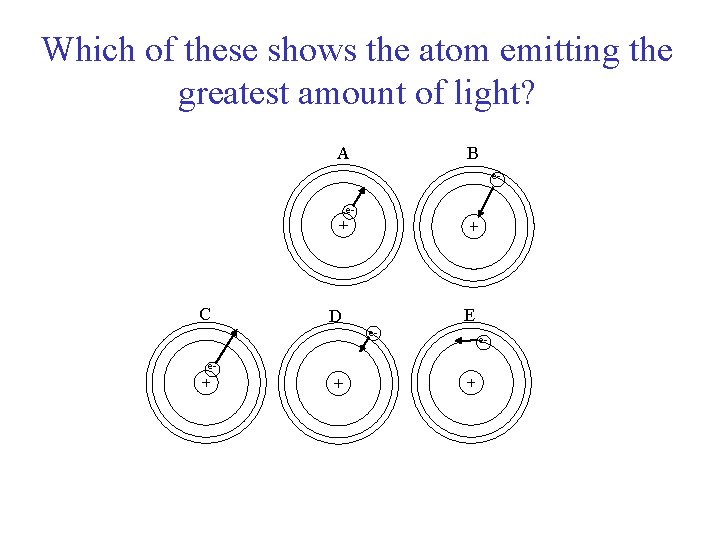
Which of these shows the atom emitting the greatest amount of light? A B e- ee- C e- D e- E e-

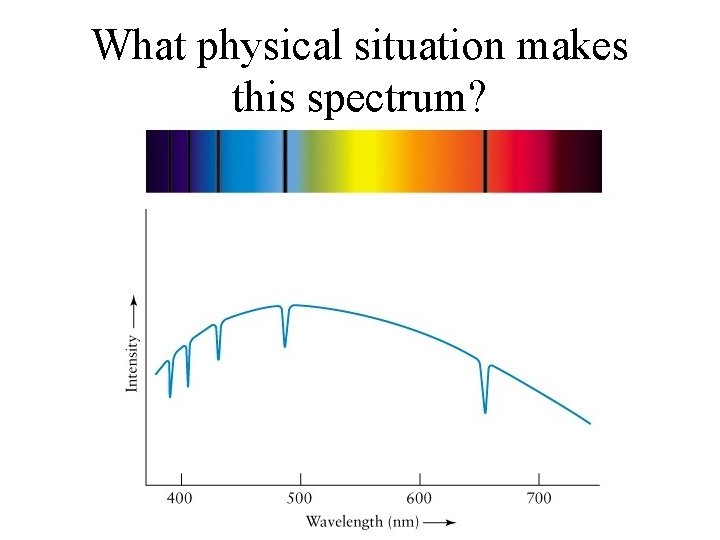
What physical situation makes this spectrum?

The type of spectrum given off depends on the objects involved Law #3 – When the light from a hot dense object passes through a cool cloud of gas, the atoms within the cloud can absorb particular colors (wavelengths) of light and produce a absorption line spectrum - a series of dark spectral lines among the colors of the rainbow.

prism Hot/Dense Energy Source Cooler low density cloud of Gas Absorption Line Spectrum

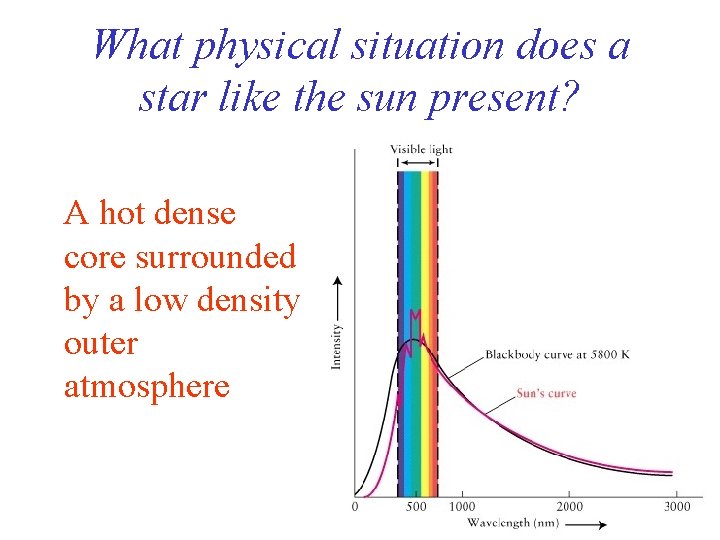
What physical situation does a star like the sun present? A hot dense core surrounded by a low density outer atmosphere

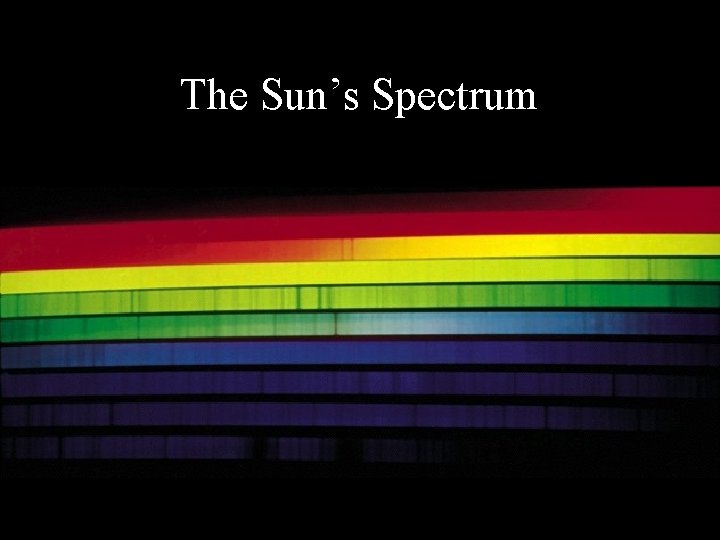
The Sun’s Spectrum

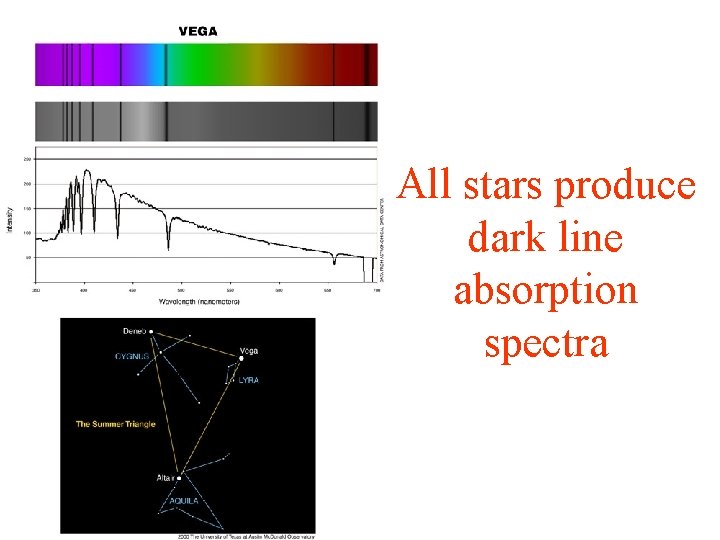
All stars produce dark line absorption spectra

What can we learn by analyzing starlight? • A star’s temperature • A star’s chemical composition

Tutorial: Analyzing Spectra – p. 43 • Work with a partner! • Read the instructions and questions carefully. • Discuss the concepts and your answers with one another. Take time to understand it now!!!! • Come to a consensus answer you both agree on. • If you get stuck or are not sure of your answer, ask another group.

Consider the dark line absorption spectra shown below for Star X and Star Z. What can you determine about the color of the two stars? Assume that the left end of each spectrum corresponds to shorter wavelengths (blue light) and that the right end of each spectrum corresponds with longer wavelengths (red light). Star X Star Z 1. Star X would appear blue and Star Z would appear red. 2. Star X would appear red and Star Z would appear blue. 3. Both stars would appear the same color. 4. The color of the stars cannot be determined from this information.
 Light light light chapter 23
Light light light chapter 23 Into the light chapter 22
Into the light chapter 22 Chapter 22
Chapter 22 Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Nature and propagation of light
Nature and propagation of light Put out that light
Put out that light Membrane-bound organelles
Membrane-bound organelles The bouncing off of light
The bouncing off of light Nature nature controversy
Nature nature controversy Opcvl
Opcvl Opcvl
Opcvl Nature of light wave
Nature of light wave Wavestown answers
Wavestown answers What is meant by the dual wave particle nature of light
What is meant by the dual wave particle nature of light How to find the height of an image in a convex mirror
How to find the height of an image in a convex mirror Dual nature of light
Dual nature of light Chapter 27 quantum theory study guide answers
Chapter 27 quantum theory study guide answers Dual nature of light
Dual nature of light Material blocks light
Material blocks light Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi
Hát lên người ơi Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên