The need for Pharmacovigilance Shanthi Pal Quality Assurance

























- Slides: 36

The need for Pharmacovigilance Shanthi Pal Quality Assurance and Safety of Medicines 1| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

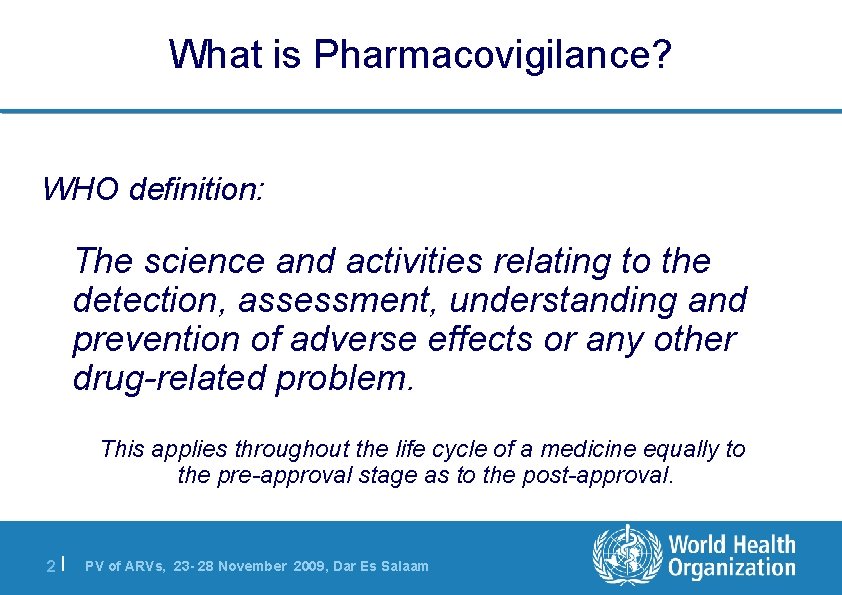
What is Pharmacovigilance? WHO definition: The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. This applies throughout the life cycle of a medicine equally to the pre-approval stage as to the post-approval. 2| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

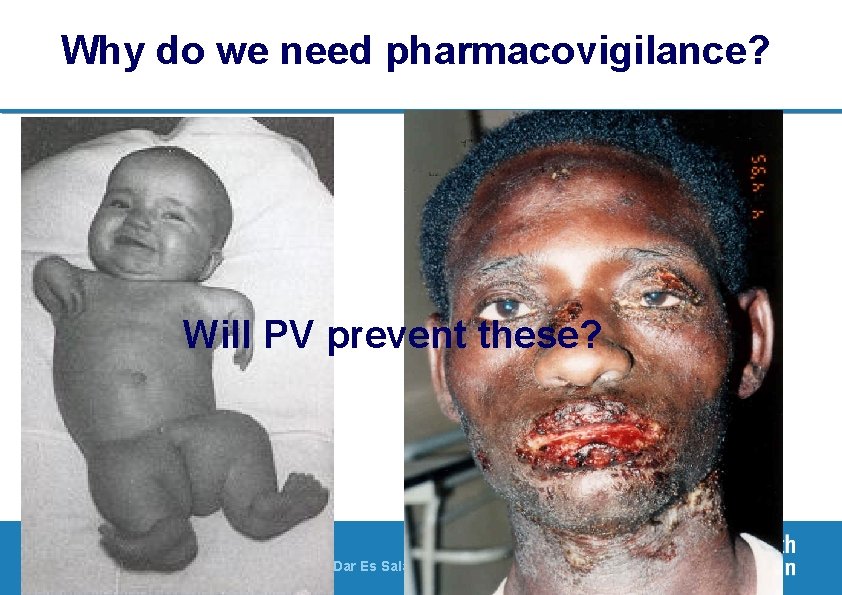
Why do we need pharmacovigilance? Will PV prevent these? 3| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

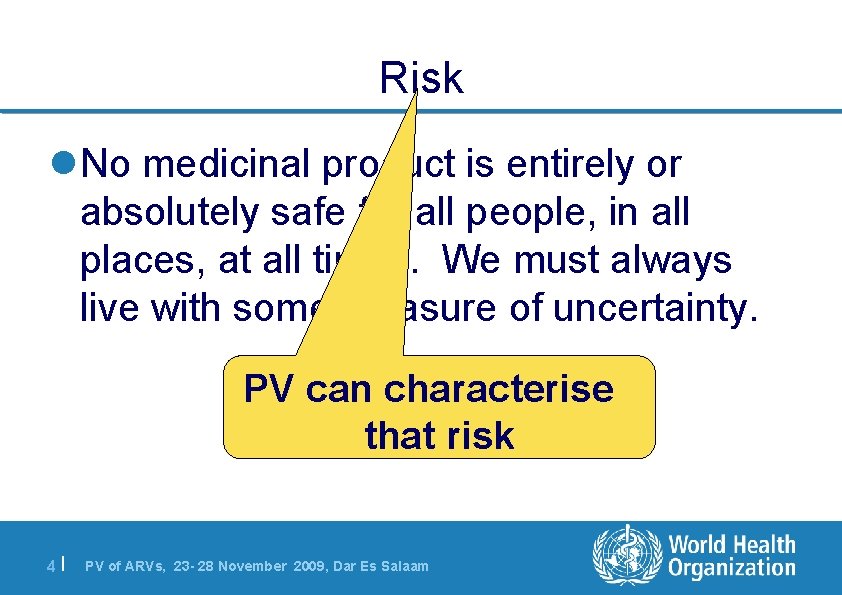
Risk l. No medicinal product is entirely or absolutely safe for all people, in all places, at all times. We must always live with some measure of uncertainty. PV can characterise that risk 4| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Why do we need pharmacovigilance? Ten reasons why…. 5| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Why do we need pharmacovigilance? Reason 1: l Insufficient evidence of safety from clinical trials 6| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

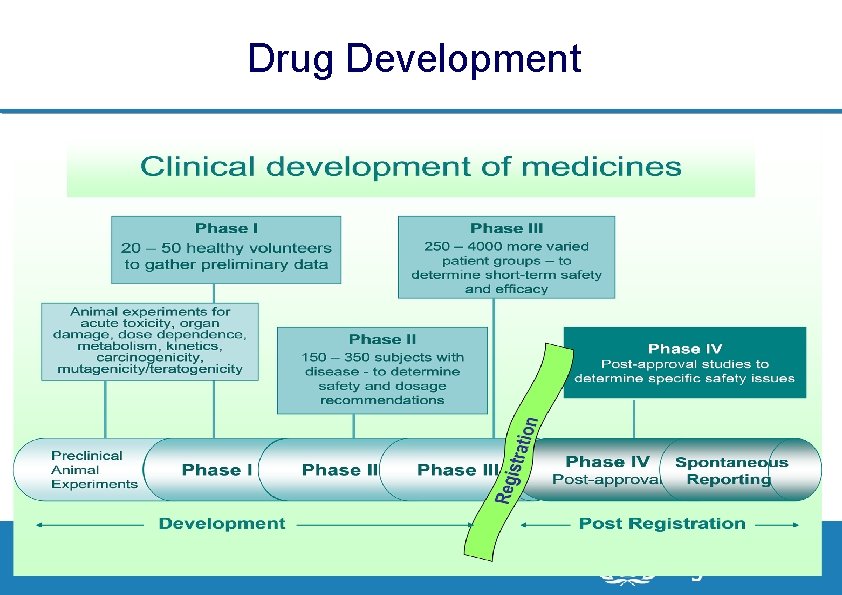
Drug Development 7| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

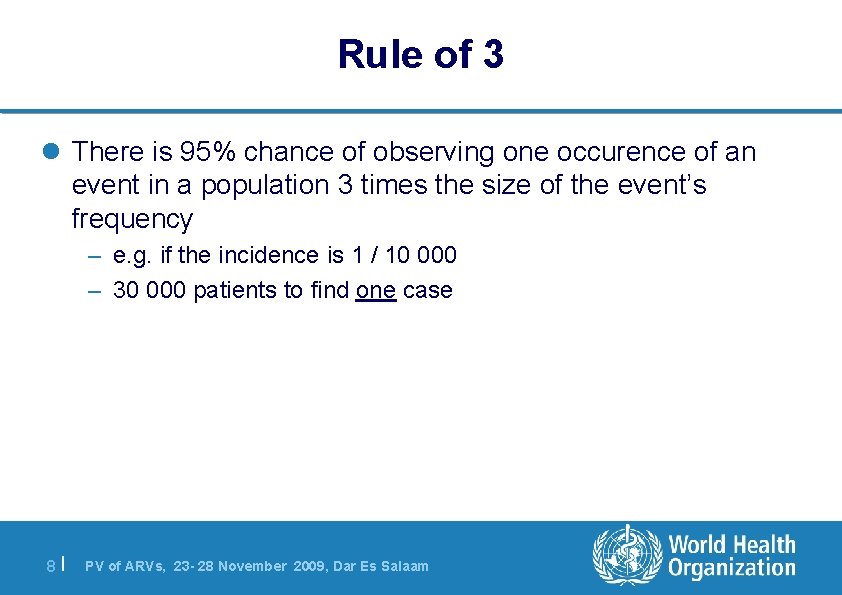
Rule of 3 l There is 95% chance of observing one occurence of an event in a population 3 times the size of the event’s frequency – e. g. if the incidence is 1 / 10 000 – 30 000 patients to find one case 8| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

'other' limitations of phase 1 -3 clinical trials l narrow population: age and sex specific l narrow indications: only the specific disease studied l short duration: often no longer than a few weeks 9| PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 2 Medicines are supposed to save lives Dying from a disease is sometimes unavoidable; dying from a medicine is unacceptable. Lepakhin V. Geneva 2005 10 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

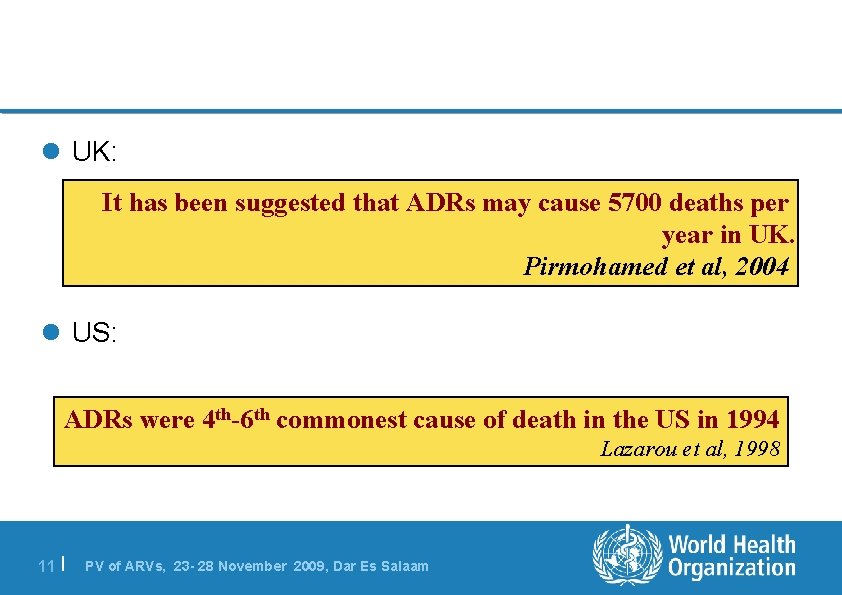
l UK: It has been suggested that ADRs may cause 5700 deaths per year in UK. Pirmohamed et al, 2004 l US: ADRs were 4 th-6 th commonest cause of death in the US in 1994 Lazarou et al, 1998 11 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 3 l To KEEP products on the market 12 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Examples of product recalls due to toxicity l Medicine Year l Examples of serious and unexpected adverse events leading to withdrawal of medicine l Thalidomide 1965 l Phocomelia l Practolol 1975 l Sclerosing peritonitis l Clioquinol 1970 l Subacute nephropathy l Benoxaprofen 1982 l Nephrotoxicity, cholestatic jaundice l Terfenadine 1997 l Torsade de pointes l Rofecoxib 2004 l Cardiovascular effects l Veralipride 2007 l Anxiety, depression, movement disorders 13 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

But… Is product recall the aim of PV? 14 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

No because. . . l No drug is inherently safe – unless it has no effect at all! if patients do well, so will the drugs l Each(but patient is unique not necessarily the other way around!) l Each treatment situation is unique – What is the right drug for me might be a bad choice for you l Understanding this will help make the right choice for each patient 15 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 4 To protect patients from unnecessary harm Many ADRs are preventable 16 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

l 125 Patients l 24 Patients experienced ADRs (19%) (59%) were avoidable 17 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

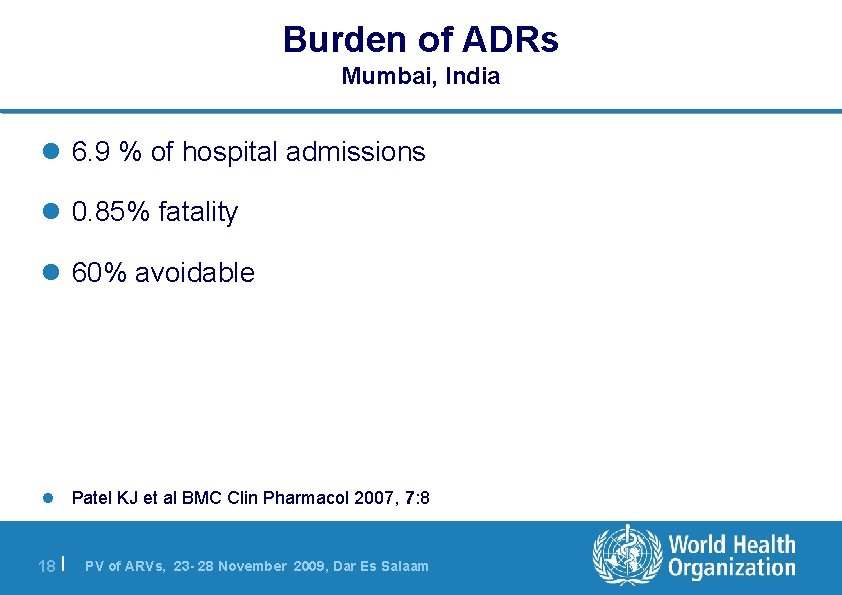
Burden of ADRs Mumbai, India l 6. 9 % of hospital admissions l 0. 85% fatality l 60% avoidable l Patel KJ et al BMC Clin Pharmacol 2007, 7: 8 18 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

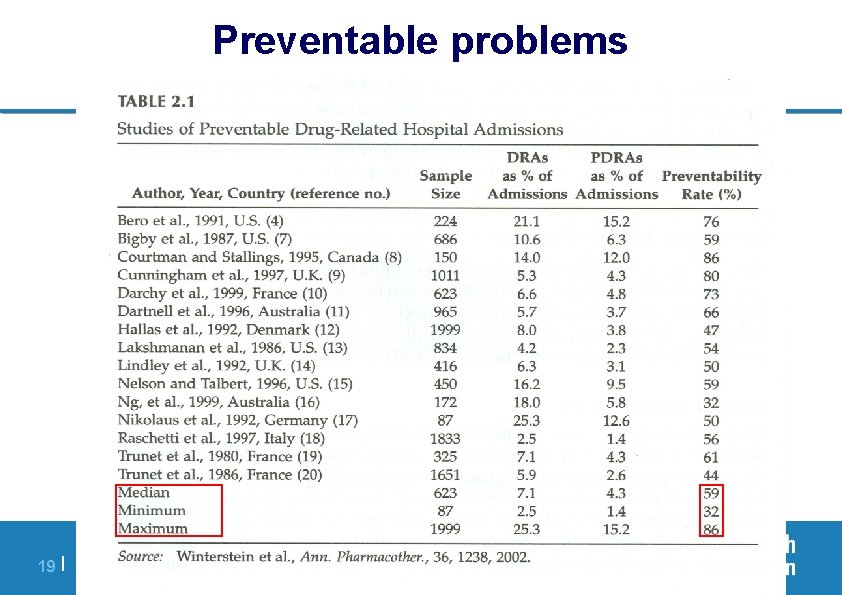
Preventable problems 19 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 5 To reduce healthcare expenses ADRs are a huge burden !! 20 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

l 6. 5% of admissions are due to ADRs l Seven 800 -bed hospitals are occupied by ADR patients Cost £ 446 million per annum 21 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Cost of ADRs in the US? l Cost of drug related morbidity and mortality exceeded $177. 4 billion in 2000 (Ernst FR & Grizzle AJ, 2001: J American Pharm. Assoc) l ADR related cost to the country exceeds the cost of the medications themselves 22 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

l More recent data from EU as a whole Cost due to ADRs in EU: € 79 billions/year Ref: Press Release from Brussels, 10 Dec 2008. 23 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Cost due to ADRs Mumbai, India l Additional cost to hospital INR 6197/patient (US$150) l Patel KJ et al BMC Clin Pharmacol 2007, 7: 8 24 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

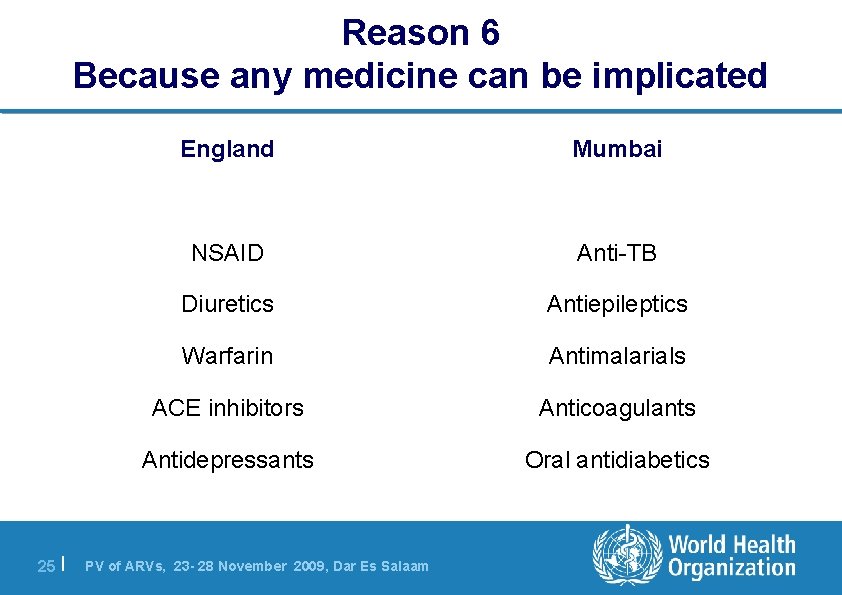
Reason 6 Because any medicine can be implicated 25 | England Mumbai NSAID Anti-TB Diuretics Antiepileptics Warfarin Antimalarials ACE inhibitors Anticoagulants Antidepressants Oral antidiabetics PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 7 Promoting rational use of medicines and adherence 26 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Prescription Dr A. Who 31 December 2000 Re: Mr Joseph Bloggs 1) abacavir + lamivudine + zidovudine 1 BD 2) atenolol 100 mg/d 3) acetylsalicylic acid 150 mg/d 4) cerivastatin 10 mg/d 5) gemfibrozil 200 mg/d 6) metformin 500 mg/d 7) fluoxetine 50 mg/d 8) Sildenafil 27 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

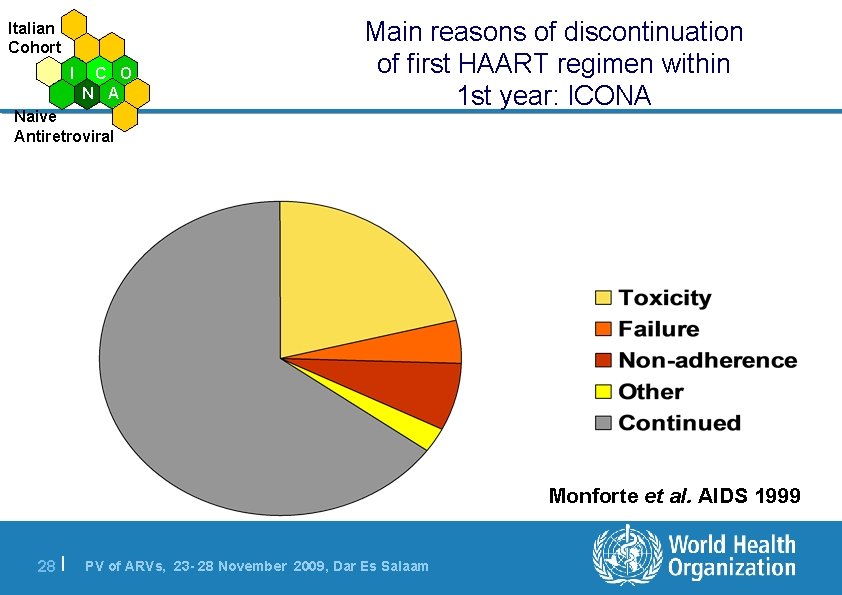
Italian Cohort I C O N A Naive Antiretroviral Main reasons of discontinuation of first HAART regimen within 1 st year: ICONA Monforte et al. AIDS 1999 28 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 8 Ensuring public confidence If something can go wrong, it will – Murphy's law 29 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

30 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 9 Ethical thing to do To know of something that is harmful to another person who does not know, and not telling, is unethical 31 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

l Not reporting a serious unknown reaction is unethical valid for everyone • patient • health professional • manufacturer • authorities 32 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Consequence ALLEGATION: ! ! r e t disas Known about SSRI prescribing at unsafe doses for a decade Guardian Weekly March 18 -24 2004 33 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Reason 10 It can unveil lapses in BEST PRACTICES l Unexpected lack of effect – counterfeiting – resistance – interaction l Quality problems l Dependence and abuse l Poisoning l Medication errors 34 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Pharmacovigilance Major Aims l early detection of unknown safety problems l detection of increases in frequency l identification of risk factors l quantifying risks l communicating information l preventing patients from being affected unnecessarily Rational and Safe use of Medicines 35 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam

Pharmacovigilance is Essential 36 | PV of ARVs, 23 - 28 November 2009, Dar Es Salaam
 Pharmacovigilance quality assurance
Pharmacovigilance quality assurance Shanti raghavan enable india
Shanti raghavan enable india Performance status
Performance status Quality improvement vs quality assurance
Quality improvement vs quality assurance Project quality management pmp
Project quality management pmp Quality assurance vs quality control
Quality assurance vs quality control Pmbok quality assurance vs quality control
Pmbok quality assurance vs quality control Process of nursing audit
Process of nursing audit Quality assurance vs quality control
Quality assurance vs quality control Pal assurance
Pal assurance Cem stands for in pharmacovigilance
Cem stands for in pharmacovigilance Pvnet pharmacovigilance
Pvnet pharmacovigilance Objectives of pharmacovigilance
Objectives of pharmacovigilance Aims of pharmacovigilance
Aims of pharmacovigilance Application of pharmacovigilance in zambia
Application of pharmacovigilance in zambia Netherlands pharmacovigilance centre lareb
Netherlands pharmacovigilance centre lareb International pharmacovigilance centre
International pharmacovigilance centre Adrereport
Adrereport Ainlp
Ainlp Solicited reports in pharmacovigilance
Solicited reports in pharmacovigilance Pharmacovigilance signal detection methods
Pharmacovigilance signal detection methods Pharmacovigilance ppt
Pharmacovigilance ppt Kålbrok
Kålbrok Principles of pharmacovigilance
Principles of pharmacovigilance Cem in pharmacovigilance
Cem in pharmacovigilance Pharmacovigilance compliance
Pharmacovigilance compliance Youtube.com
Youtube.com Software quality assurance agency uk
Software quality assurance agency uk Nursing quality assurance commission
Nursing quality assurance commission Aviation maintenance quality management
Aviation maintenance quality management European quality assurance standards
European quality assurance standards Quality revolution in software testing
Quality revolution in software testing Delivery quality assurance
Delivery quality assurance Quality assurance definition
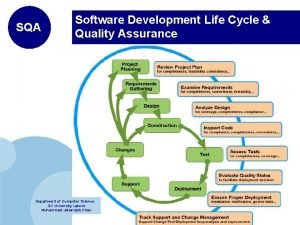
Quality assurance definition Sqa life cycle
Sqa life cycle Statistical software quality assurance
Statistical software quality assurance Packaging quality assurance
Packaging quality assurance