Pharmacovigilance Shanthi Pal M Pharm Ph D Quality




















- Slides: 24

Pharmacovigilance Shanthi Pal, M. Pharm, Ph. D Quality Assurance and Safety of Medicines WHO 1 World Health Organization

Objectives • To discuss the need for pharmacovigilance • To present WHO’s role in promoting pharmacovigilance 2 World Health Organization

Medicine Safety • To undergo treatment you have to be very healthy, because apart from your sickness you have to stand the medicine. Molière 3 World Health Organization

Pharmacovigilance What IS this? 4 World Health Organization

Vigilance Vigilare = to watch alert watchfulness forbearance of sleep; wakefulness watchfulness in respect of danger; care; caution; circumspection the process of paying close and continuous attention 5 World Health Organization

Pharmacovigilance • The science and activities relating to the detection, evaluation, understanding and prevention of adverse drug reactions or any other drug-related problems 6 World Health Organization

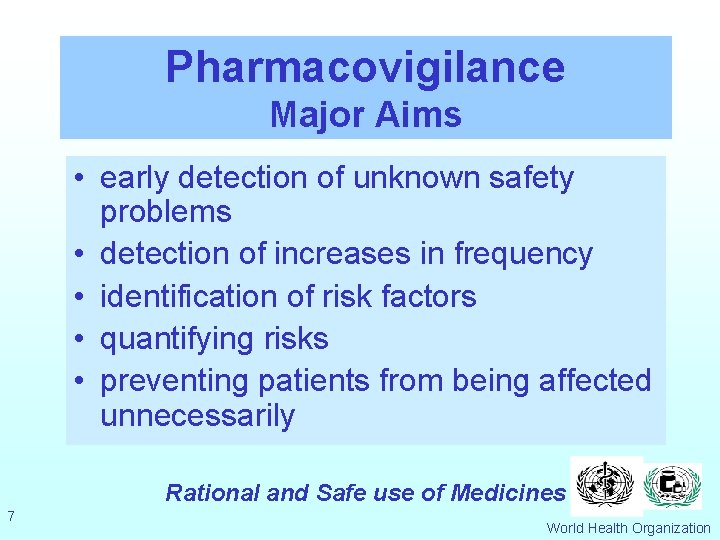
Pharmacovigilance Major Aims • early detection of unknown safety problems • detection of increases in frequency • identification of risk factors • quantifying risks • preventing patients from being affected unnecessarily Rational and Safe use of Medicines 7 World Health Organization

Why Pharmacovigilance? 8 World Health Organization

Why Pharmacovigilance? • Post-marketing Topics Unexpected adverse reactions Interactions Dependence Long-term efficacy, Resistance Risk factors Quality (Counterfeit) Cost assessment 9 World Health Organization

Why Pharmacovigilance? • Adverse Drug Reactions are the 4 th to 6 th largest cause of mortality in the USA (Lazarou J. et al. , 1998) 10 World Health Organization

Why Pharmacovigilance? The percentage of hospital admissions due to drug related events in some countries is about or more than 10%. • UK Study : 10. 1 % (Bhalla et al, 2003) • French study : 10. 3 % prevalence of ADRs (Imbs et al, 1999) 11 World Health Organization

Why Pharmacovigilance? Economic impact Drug related morbidity and mortality expenses exceeded US$ 177. 4 billion in the USA in 2000 (Ernst & Grizzle, 2001) 12 World Health Organization

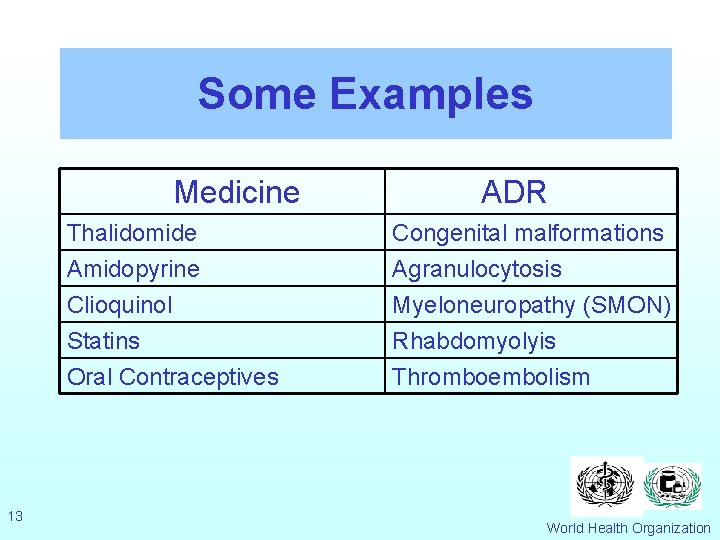
Some Examples Medicine 13 ADR Thalidomide Amidopyrine Clioquinol Congenital malformations Agranulocytosis Myeloneuropathy (SMON) Statins Oral Contraceptives Rhabdomyolyis Thromboembolism World Health Organization

14 World Health Organization

WHO Programme for International Drug Monitoring (HQ) • • 15 Policy Exchange of Information Technical support to countries Advisory Committee on Safety of Medicinal Products World Health Organization

Exchange of Information • • 16 WHO Pharmaceuticals Newsletter WHO Drug Alerts WHO Drug Information WHO Restricted Pharmaceuticals List (Vigimed - electronic exchange) (Uppsala Reports) (Signal) World Health Organization

Technical support to countries • Technical guidelines on all aspects of pharmacovigilance (Several publications and documents) • Training courses on pharmacovigilance (Regional Training Courses, biennial course by UMC and HQ) 17 World Health Organization

Training courses for Public Health Programmes • Introducing Pharmacovigilance into Malaria Programmes- Zambia 2003 • Introducing Pharmacovigilance into HIV/AIDS Programmes – South Africa 2004 18 World Health Organization

19 World Health Organization

WHO Collaborating Centre (Uppsala Monitoring Centre) ADR database • No of reports: more than 3 million • Each year increase ~250, 000 / year • Top 5 reporting countries • • • 20 USA United Kingdom Germany Australia Canada World Health Organization

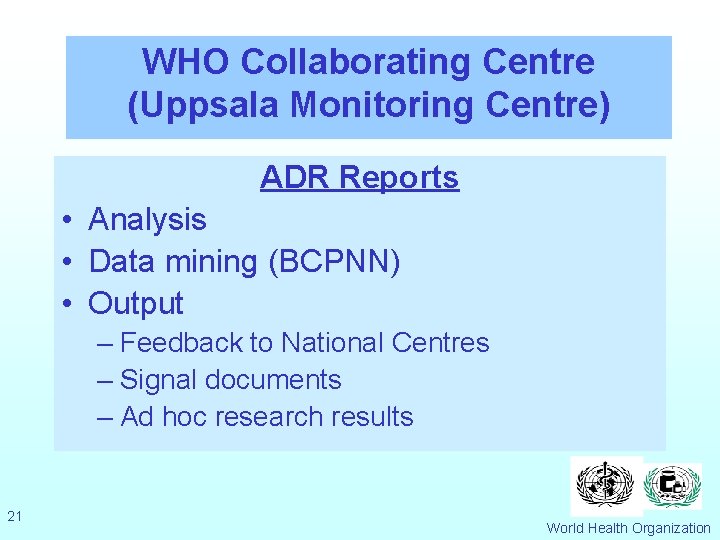
WHO Collaborating Centre (Uppsala Monitoring Centre) ADR Reports • Analysis • Data mining (BCPNN) • Output – Feedback to National Centres – Signal documents – Ad hoc research results 21 World Health Organization

Future challenges • • • 22 Raise awareness Monitor all medicines Address broader safety concerns Integrate work throughout WHO Improve training activities World Health Organization

In conclusion …. • The work of WHO in the area of safety monitoring of medicines is necessary if we are to achieve the mission of EDM: • Medicines should be Available, Affordable, Safe and Properly used. 23 World Health Organization

Thank you ' A blue fly, if it clings to the tail of a thoroughbred horse, can travel ten thousand miles '. 24 World Health Organization