Pharmacovigilance challenges facing WHO Shanthi Pal Leader Medicines







- Slides: 15

Pharmacovigilance: challenges facing WHO Shanthi Pal Leader, Medicines Safety and Vigilance, WHO, Geneva

What is pharmacovigilance • The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problems. (The Importance of Pharmacovigilance, WHO 2002) • A tool for generating evidence to influence policies

WHO Global PV Programme Why? ? • World Health Assembly Resolution 16. 36 • INVITES Member States to arrange for a systematic collection of information on serious adverse drug reactions observed during the development of a drug and, in particular, after its release for general use.

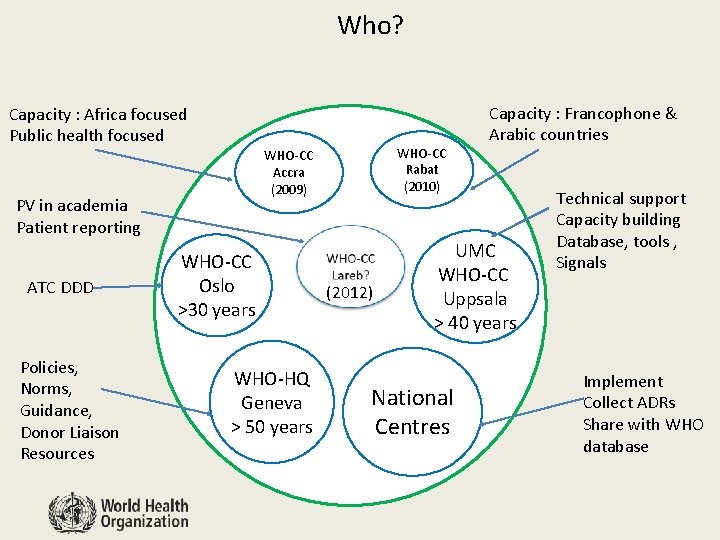
Who? Capacity : Africa focused Public health focused PV in academia Patient reporting ATC DDD Policies, Norms, Guidance, Donor Liaison Resources WHO-CC Rabat (2010) WHO-CC Accra (2009) WHO-CC Oslo >30 years WHO-HQ Geneva > 50 years (2012) Capacity : Francophone & Arabic countries UMC WHO-CC Uppsala > 40 years National Centres Technical support Capacity building Database, tools , Signals Implement Collect ADRs Share with WHO database

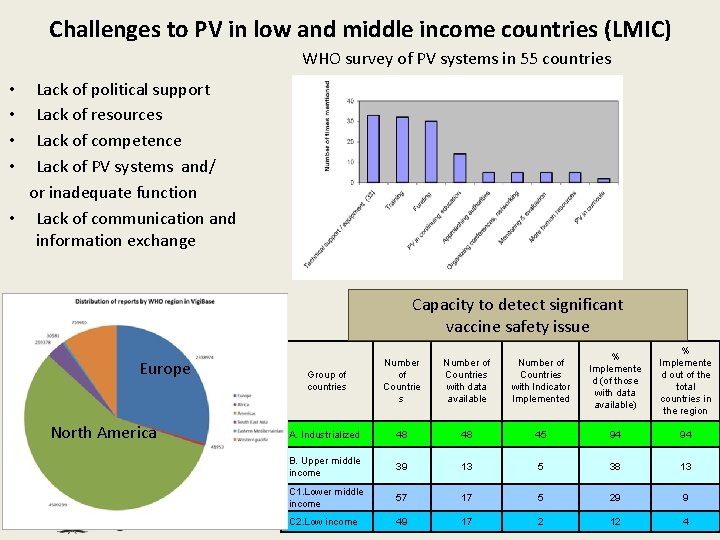
Challenges to PV in low and middle income countries (LMIC) WHO survey of PV systems in 55 countries Lack of political support Lack of resources Lack of competence Lack of PV systems and/ or inadequate function • Lack of communication and information exchange • • Capacity to detect significant vaccine safety issue Europe North America Group of countries Number of Countries with data available Number of Countries with Indicator Implemented % Implemente d (of those with data available) % Implemente d out of the total countries in the region A. Industrialized 48 48 45 94 94 B. Upper middle income 39 13 5 38 13 C 1. Lower middle income 57 17 5 29 9 C 2. Low income 49 17 2 12 4

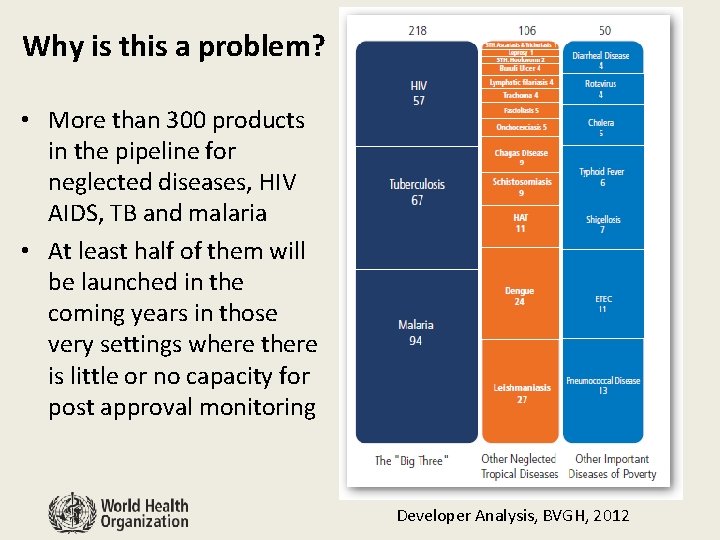
Why is this a problem? • More than 300 products in the pipeline for neglected diseases, HIV AIDS, TB and malaria • At least half of them will be launched in the coming years in those very settings where there is little or no capacity for post approval monitoring Developer Analysis, BVGH, 2012

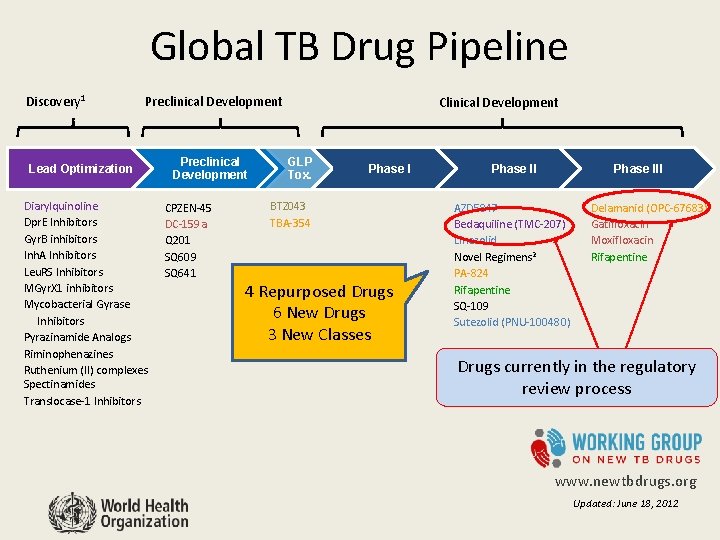
Global TB Drug Pipeline Discovery 1 Preclinical Development Lead Optimization Diarylquinoline Dpr. E Inhibitors Gyr. B inhibitors Inh. A Inhibitors Leu. RS Inhibitors MGyr. X 1 inhibitors Mycobacterial Gyrase Inhibitors Pyrazinamide Analogs Riminophenazines Ruthenium (II) complexes Spectinamides Translocase-1 Inhibitors Preclinical Development CPZEN-45 DC-159 a Q 201 SQ 609 SQ 641 Clinical Development GLP Tox. Phase I BTZ 043 TBA-354 4 Repurposed Drugs 6 New Drugs 3 New Classes Phase III AZD 5847 Bedaquiline (TMC-207) Linezolid Novel Regimens 2 PA-824 Rifapentine SQ-109 Sutezolid (PNU-100480) Delamanid (OPC-67683) Gatifloxacin Moxifloxacin Rifapentine Drugs currently in the regulatory review process www. newtbdrugs. org Updated: June 18, 2012

What can we do about it? http: //www. who. int/medicines/areas/quality_safety/safety_efficacy/saf_pub /en/

Build PV systems

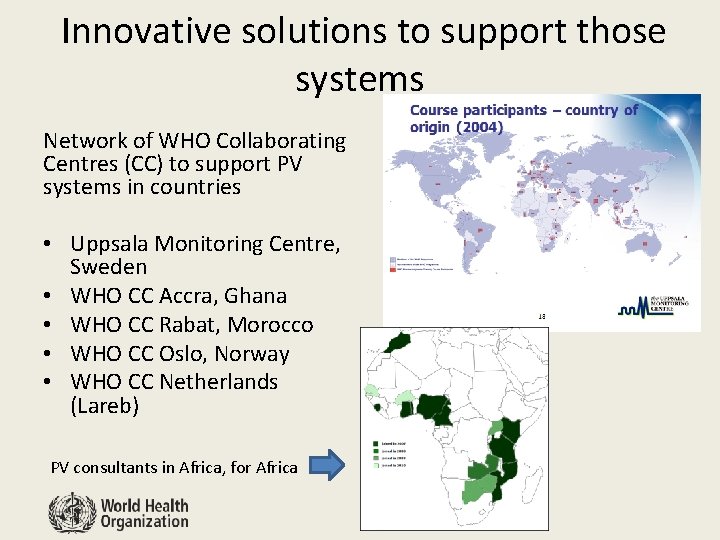
Innovative solutions to support those systems Network of WHO Collaborating Centres (CC) to support PV systems in countries • Uppsala Monitoring Centre, Sweden • WHO CC Accra, Ghana • WHO CC Rabat, Morocco • WHO CC Oslo, Norway • WHO CC Netherlands (Lareb) PV consultants in Africa, for Africa

Dedicated resources

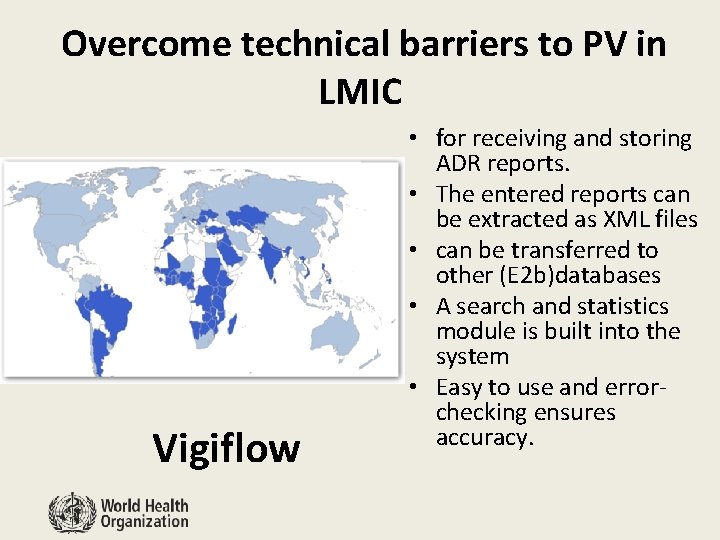
Overcome technical barriers to PV in LMIC Vigiflow • for receiving and storing ADR reports. • The entered reports can be extracted as XML files • can be transferred to other (E 2 b)databases • A search and statistics module is built into the system • Easy to use and errorchecking ensures accuracy.

Task shifting: patients as partners • Conventional models can't work in some settings • All hands on board – Task shifting • Patient reporting (more data, timely data, additional information)

Forward looking strategies through broad networks Expanding and implementing the full scope of PV Links with medication errors networks Links with SSFFC networks

Pharmacovigilance investments will yield multiple benefits • As an insurance for investments in public health interventions: – A comprehensive disease control approach must include quality and safety as a component. • By Investing in PV, countries will have data on medicines – To assist decision making by regulators, improve treatment strategies, health care practices, and treatment outcomes Pharmacovigilance data can guide procurement of effective medicines and reduce wastage • Robust safety monitoring also provides a quality assurance mechanism and helps monitor programmatic implementation. •
 Shanti raghavan enable india
Shanti raghavan enable india Performance status
Performance status Contemporary business world
Contemporary business world Challenges facing global managers
Challenges facing global managers What are the major challenges facing public libraries
What are the major challenges facing public libraries Challenges facing global sourcing
Challenges facing global sourcing Challenges facing global managers
Challenges facing global managers What are the challenges facing the church today
What are the challenges facing the church today Challenges facing family medicine
Challenges facing family medicine Transformational vs transformative leadership
Transformational vs transformative leadership Pharmacovigilance quality assurance
Pharmacovigilance quality assurance Application of pharmacovigilance in zambia
Application of pharmacovigilance in zambia Lareb netherlands
Lareb netherlands International pharmacovigilance centre
International pharmacovigilance centre Adrereport
Adrereport Ainlp
Ainlp




























