Pharmacovigilance Shanthi Pal M Pharm Ph D Quality

























- Slides: 28

Pharmacovigilance Shanthi Pal, M. Pharm, Ph. D Quality Assurance and Safety of Medicines WHO 1 World Health Organization

Medicine Safety • To undergo treatment you have to be very healthy, because apart from your sickness you have to stand the medicine. Molière 2 World Health Organization

Pharmacovigilance What IS this? 3 World Health Organization

Pharmacovigilance • The science and activities relating to the detection, evaluation, understanding and prevention of adverse drug reactions or any other drug-related problems 4 World Health Organization

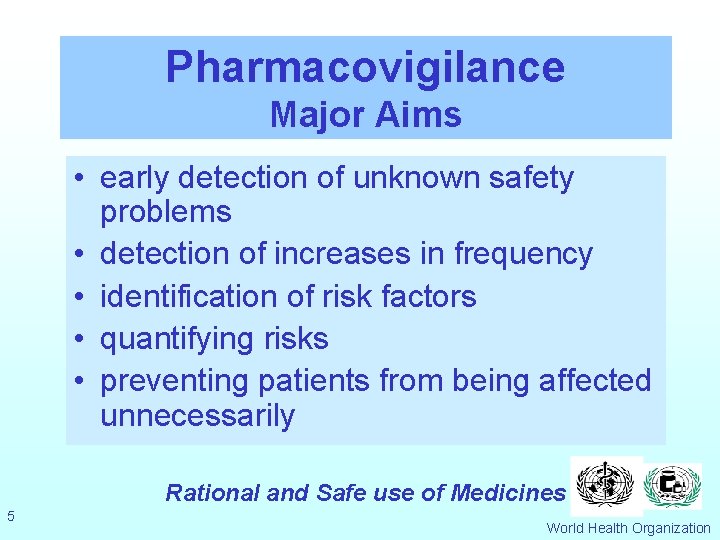
Pharmacovigilance Major Aims • early detection of unknown safety problems • detection of increases in frequency • identification of risk factors • quantifying risks • preventing patients from being affected unnecessarily Rational and Safe use of Medicines 5 World Health Organization

Why Pharmacovigilance? 6 World Health Organization

Why Pharmacovigilance? • Post-marketing Topics Unexpected adverse reactions Interactions Dependence Long-term efficacy, Resistance Risk factors Quality (Counterfeit) Cost assessment 7 World Health Organization

Why Pharmacovigilance? • Adverse Drug Reactions are the 4 th to 6 th largest cause of mortality in the USA (Lazarou J. et al. , 1998) 8 World Health Organization

Why Pharmacovigilance? The percentage of hospital admissions due to drug related events in some countries is about or more than 10%. • UK Study : 10. 1 % (Bhalla et al, 2003) • French study : 10. 3 % prevalence of ADRs (Imbs et al, 1999) 9 World Health Organization

Why Pharmacovigilance? Economic impact Drug related morbidity and mortality expenses exceeded US$ 177. 4 billion in the USA in 2000 (Ernst & Grizzle, 2001) 10 World Health Organization

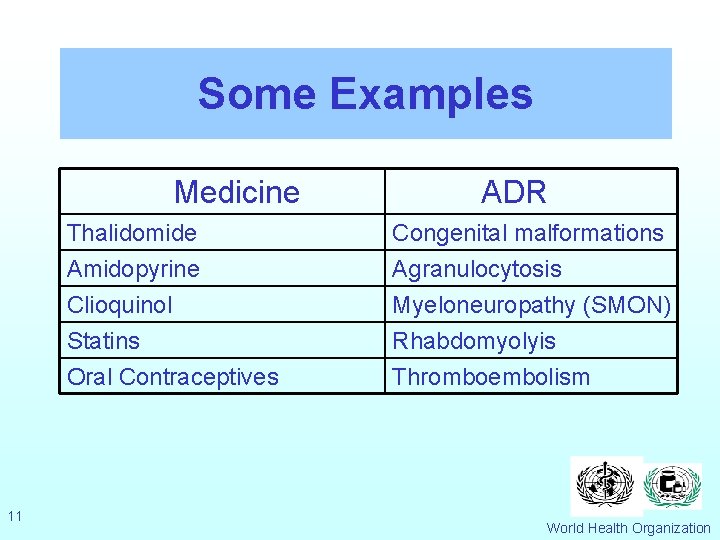
Some Examples Medicine 11 ADR Thalidomide Amidopyrine Clioquinol Congenital malformations Agranulocytosis Myeloneuropathy (SMON) Statins Oral Contraceptives Rhabdomyolyis Thromboembolism World Health Organization

Pilot project of ten countries • Australia, Canada, Denmark, Germany, Ireland, Netherlands, New Zealand, Sweden, United Kingdom, USA 12 World Health Organization

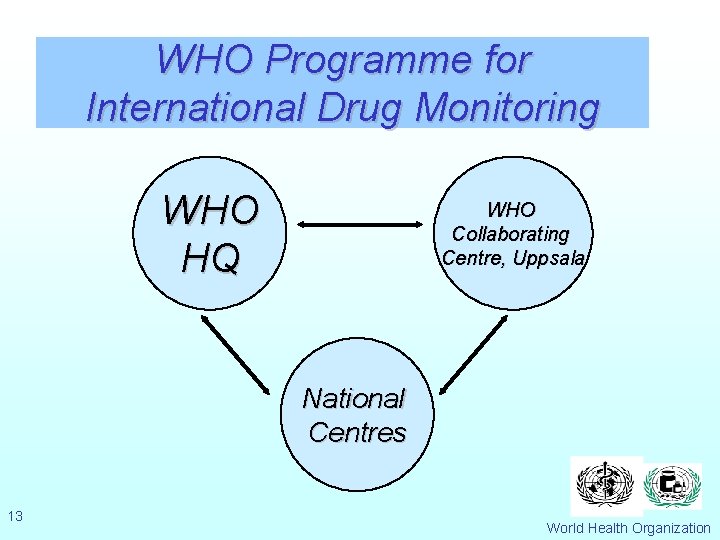
WHO Programme for International Drug Monitoring WHO HQ WHO Collaborating Centre, Uppsala National Centres 13 World Health Organization

y t e f a S saves Mary Caroline Shanthi 14 World Health Organization

Pharmacovigilance in WHO • Exchange of Information • Policies, guidelines, normative activities • Country support • Collaborations 9/9/2020 World Health Organization

Exchange of Information 16 • 24 th WHA (1971) Resolution WHA 24. 56 Need for a system of collection and dissemination of information on results of safety and effectiveness trials of new drugs and on their registration in the countries, for possible use of data by health authorities of countries importing these drugs World Health Organization

Exchange of Information INTERNATIONAL INFORMATION SYSTEM • National Information Officers (WHO Liaison Officers) • WHO Pharmaceuticals Newsletter • WHO Restricted List Updates 17 World Health Organization

Exchange of Information • Ad hoc “Drug Alerts” • WHO Drug Information • International Conference of Drug Regulatory Authorities (ICDRA) • www. who. int/medicines/edmtopics. shtml 9/9/2020 World Health Organization

Policies, Guidelines and Normative Activities • Guidelines – The Importance of Pharmacovigilance (2002) – Safety Reporting - A guide to detecting and reporting adverse drug reactions (2002) – Pharmacovigilance in public health – Safety monitoring of herbal medicines 9/9/2020 – Expert Advisory Committee World Health Organization

20 World Health Organization

Country Support • Strengthen spontaneous reporting systems • Establish active surveillance component in public health programmes • Work with the WHO Collaborating Centre for International Drug Monitoring (the Uppsala Monitoring Centre) • Ongoing Regional projects: – “Implementation and Development of Pharmacovigilance in Newly Independent States of Eastern Europe”, 9/9/2020 World Health Organization since 2000

Partnerships within WHO • • • 22 Malaria HIV/AIDS Patient Safety Leprosy (UK Thalidomide) Poisons and Chemicals Safety Traditional Medicines World Health Organization

Challenges • • 23 Awareness and Advocacy Public Health Programmes Geriatrics (Access improves longevity !!) Paediatrics (Learning from SSRIs) DTC advertising and Internet sales Pregnancy Patient Safety World Health Organization

Challenges • Patient Safety • Pregnancy • Fast Track & High Through-put Products • Counterfeit Drugs 24 World Health Organization

Solutions • Guidelines and Standards: PV in HIV/AIDS, Malaria, Paediatrics…. • Global Alliance for Patient Safety • Electronic discussion groups • Advocacy material • Training Tools • Databases 25 World Health Organization

In conclusion …. • The work of WHO in the area of safety monitoring of medicines is necessary if we are to achieve the mission of EDM: • Medicines should be Available, Affordable, Safe and Properly used. 26 World Health Organization

Backstage … Gaynor, Laura, Lisa 27 World Health Organization

Thank You Merci beaucoup ! World Health Organization