Pharmacovigilance Shanthi Pal M Pharmacy Ph D Quality
































- Slides: 39

Pharmacovigilance Shanthi Pal, M. Pharmacy, Ph. D Quality Assurance and Safety of Medicines WHO

Learning objectives n Participants will be aware of what pharmacovigilance is n Participants will learn why safety monitoring is important n Participants will learn what WHO is doing in pharmacovigilance n Participants will learn what they could do in pharmacovigilance 2

Medicine Safety To undergo treatment you have to be very healthy, because apart from your sickness you have to withstand the medicine. Molière 3

Pharmacovigilance What IS this?

Pharmacovigilance The science and activities relating to the detection, evaluation, understanding and prevention of adverse drug reactions or any other drug-related problems 5

Pharmacovigilance Major Aims n early detection of unknown safety problems n detection of increases in frequency n identification of risk factors n quantifying risks n preventing patients from being affected unnecessarily Rational and Safe use of Medicines 6

Why Pharmacovigilance? Pre-marketing safety data n Animal Experiments: Relevant? n Clinical Trials: Complete? 7

Why Pharmacovigilance? Post Marketing Topics n Unexpected adverse reactions n Interactions n Risk factors n Quality of life n Long-term efficacy n Cost assessment 8

Why Pharmacovigilance? Adverse Drug Reactions are among the top ten causes of mortality (Lazarou J. et al. , 1998) 9

Why Pharmacovigilance? The percentage of hospital admissions due to drug related events in some countries is about or more than 10%. (Bhalla et al, 2003; Imbs et al, 1999) 10

Why Pharmacovigilance? Economic impact Drug related morbidity and mortality expenses exceeded US$ 177. 4 billion in the USA in 2000 (Ernst & Grizzle, 2001) 11

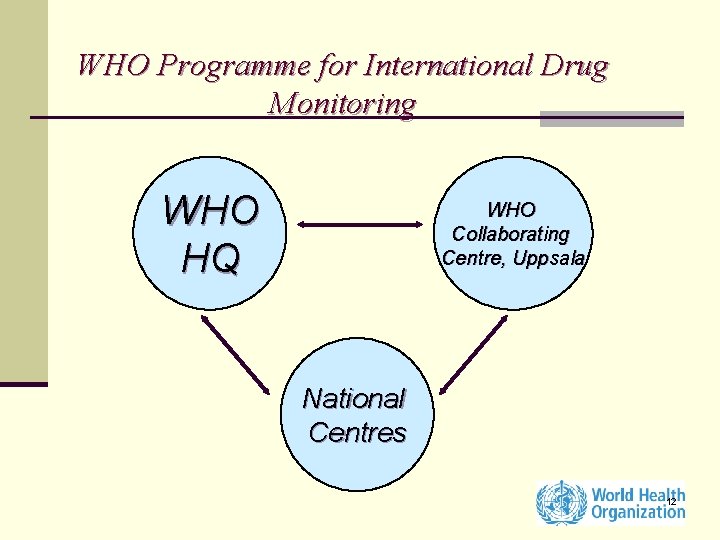
WHO Programme for International Drug Monitoring WHO HQ WHO Collaborating Centre, Uppsala National Centres 12

WHO Programme for International Drug Monitoring (HQ) n Policy n Exchange of Information n Technical support to countries n Advisory Committee on Safety of Medicinal Products 13

14

Technical support to countries n Training courses on pharmacovigilance (Regional Training Courses, biennial course by UMC and HQ) n Annual Meeting of Pharmacovigilance Centres 15

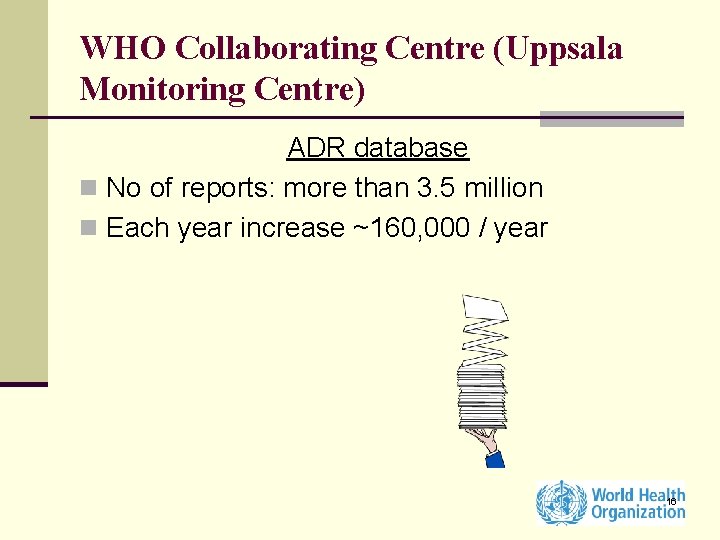
WHO Collaborating Centre (Uppsala Monitoring Centre) ADR database n No of reports: more than 3. 5 million n Each year increase ~160, 000 / year 16

WHO Collaborating Centre (Uppsala Monitoring Centre) ADR Reports n Analysis n Output n Feedback to National Centres n Signal documents 17

18

Why Pharmacovigilance for Procurement and Management Supply Plans? n It is not always the product that determines drug safety but how it is used n There is a high risk of misuse of drugs Disease Population Drug Health care system n More than 50% of ADRs are preventable 19

Public Health or community health Science and art of preventing disease, prolonging life and promoting health and efficiency through organized community efforts. 20

Public Health Programmes n Specific to each country (developed or developing) n Dependent on: The specific burden of illness The epidemiology of prevalent disease 21

DEVELOPING COUNTRIES ü Endemic and/or epidemic diseases Tuberculosis, Leprosy, HIV/AIDS, STD Malaria, Schistosomiasis, Amoebiasis, Leishmaniasis, Trachoma, Lymphatic filariasis, Onchocerciasis, ü High morbidity and mortality rates 22

PHP n Education n Environmental modifications n Nutrition intervention n Lifestyle and behavioural changes n Mass free distribution of drugs 23

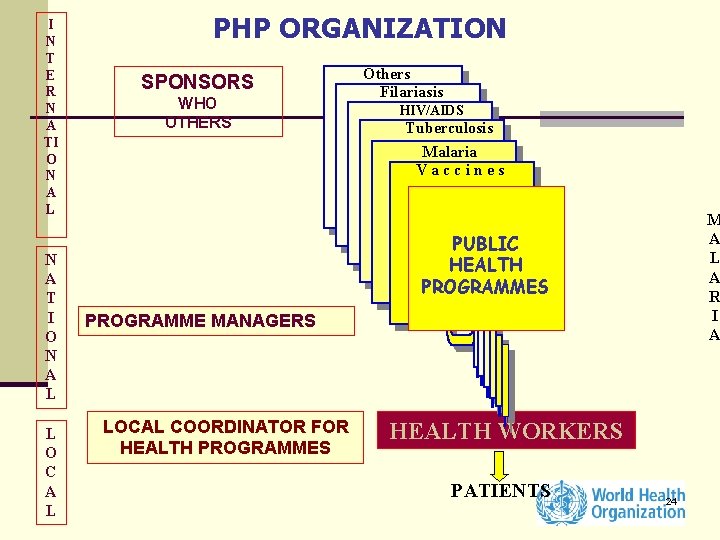
I N T E R N A TI O N A L N A T I O N A L L O C A L PHP ORGANIZATION SPONSORS WHO OTHERS Others Filariasis HIV/AIDS LEVETuberculosis L Malaria Vaccines PROGRAMME MANAGERS LOCAL COORDINATOR FOR HEALTH PROGRAMMES M A L PUBLIC A HEALTH R PROGRAMMES I A M A L A R I A HEALTH WORKERS PATIENTS 24

PHP monitoring ü Incidence and prevalence of the disease ü Morbidity and mortality rates ü Number of patients treated ü Number of drug units delivered What about the risk / effectiveness of drugs used? 25

PHP guidelines (WHO, National) Inadequate (no) reference to: n ADRs n Pharmacovigilance n Reporting 26

New Challenges in PHPs § Mass treatment regimens § Nutritional aspects § Unlabelled and off-labelled indications (pregnant or breast feeding woman, small children, elderly people) § Drug resistances § New drugs § Co-morbidities § Adherence 27

Eroding confidence in the malaria programme 28

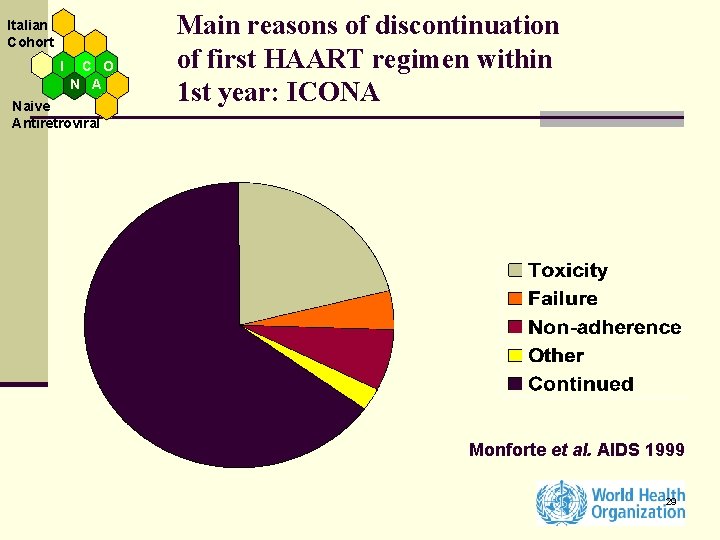
Italian Cohort I C O N A Naive Antiretroviral Main reasons of discontinuation of first HAART regimen within 1 st year: ICONA Monforte et al. AIDS 1999 29

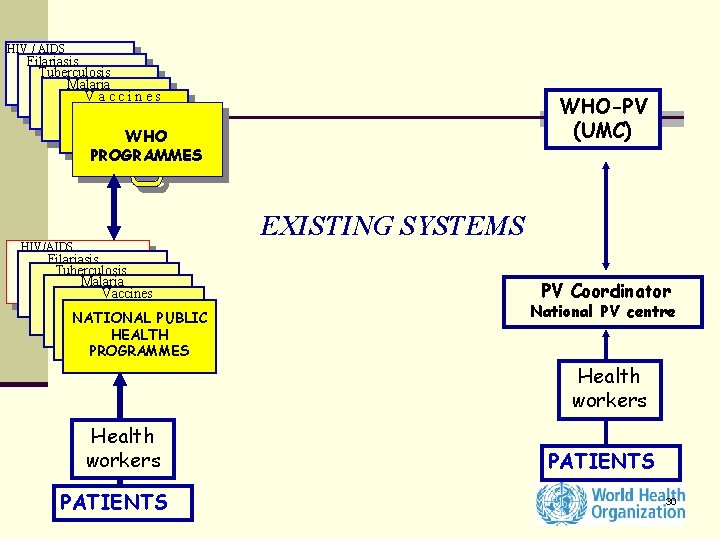
HIV / AIDS Filariasis Tuberculosis Malaria Vaccines WHO-PV (UMC) WHO PROGRAMMES EXISTING SYSTEMS HIV/AIDS Filariasis Tuberculosis Malaria Vaccines NATIONAL PUBLIC HEALTH PROGRAMMES PV Coordinator National PV centre Health workers PATIENTS 30

Urgent need for synergistic collaboration PHP PV ü detect, evaluate, and prevent adverse events ü promote rational use of drugs in activities mass treatment programmes ü Offer a cohort of patients under ü Evaluate the impact of the controlled conditions to be programmes monitored for safety over a period of time ü improve acceptability of the programme ü opportunity to implement PV 31

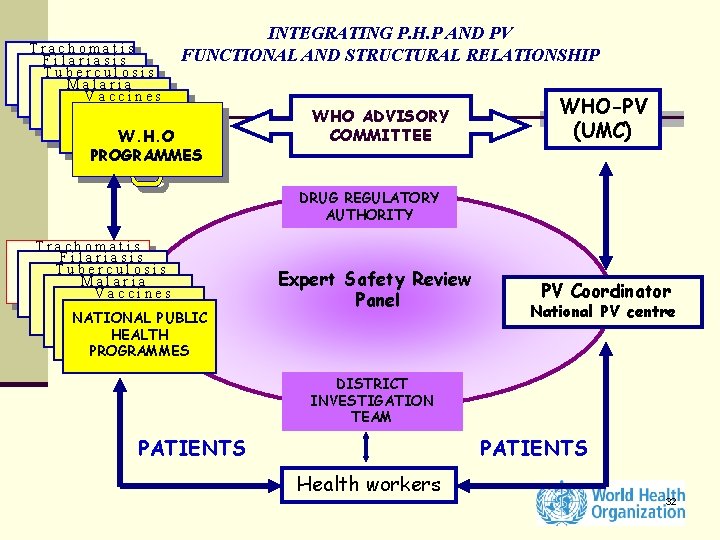
Trachomatis Filariasis Tuberculosis Malaria Vaccines INTEGRATING P. H. P AND PV FUNCTIONAL AND STRUCTURAL RELATIONSHIP W. H. O PROGRAMMES WHO ADVISORY COMMITTEE WHO-PV (UMC) DRUG REGULATORY AUTHORITY Trachomatis Filariasis Tuberculosis Malaria Vaccines NATIONAL PUBLIC HEALTH PROGRAMMES Expert Safety Review Panel PV Coordinator National PV centre DISTRICT INVESTIGATION TEAM PATIENTS Health workers 32

PV and PHP Synergy n Strengthen spontaneous reporting systems n Establish active surveillance component in public health programmes HIV/AIDS Malaria Lymphatic filariasis n Work with the WHO Collaborating Centre for International Drug Monitoring (the Uppsala Monitoring Centre) 33

Malaria Collaboration n n Joint training course Joint reviews of specific antimalarials n n Artemesinin derivatives Chlorproguanil-dapsone Amodiaquine-artesunate Joint initiatives for collaboration with pharmaceutical industry – Novartis Agreement 34

Collaboration with HIV/AIDS n Workshop in Pretoria 2004 n Action plan developed by ACSo. MP 2005 n Joint training course planned for April 2006 35

Collaboration with TDR n Chlorproguanil-dapsone example n Joint initiatives on post-marketing surveillance studies (Phase 4 clinical trials) n Joint initiatives on development of pregnancy registers for antimalarials and antrietrovirals 36

"Dying from a disease is sometimes unavoidable. But, dying from an adverse drug reaction is unacceptable". - Dr Vladimir Lepakhin Geneva 2005 37

Procurement and Supply Management Plan 2. 6 Ensuring rational use of medicines Is there a system for monitoring adverse drug reactions and drug resistance? If yes, describe briefly how the system works. If no, describe plans to establish a system. 38

Thank You Merci beaucoup !