The maintenance of a relatively constant internal environment


















- Slides: 39

The maintenance of a relatively constant internal environment in is termed: A. Positive Feedback B. Homeostasis C. Negative Feedback D. Homeopathy E. Osmosis

Which of the following is an organ? A. Mitochondria B. Blood C. Fat D. Skin E. Cardiac Muscle

A collection of cells that work together to perform a function is termed a(n): A. Organelle B. Organ C. Cell D. Tissue E. Prison

Chemistry of Life I. Properties of Atoms II. Chemical Bonds III. Reactions

Why do I have to learn Chemistry? Answer: Physiology is applied chemistry Topics requiring knowledge of chemistry: - metabolism - nerve impulses - blood p. H - protein structure

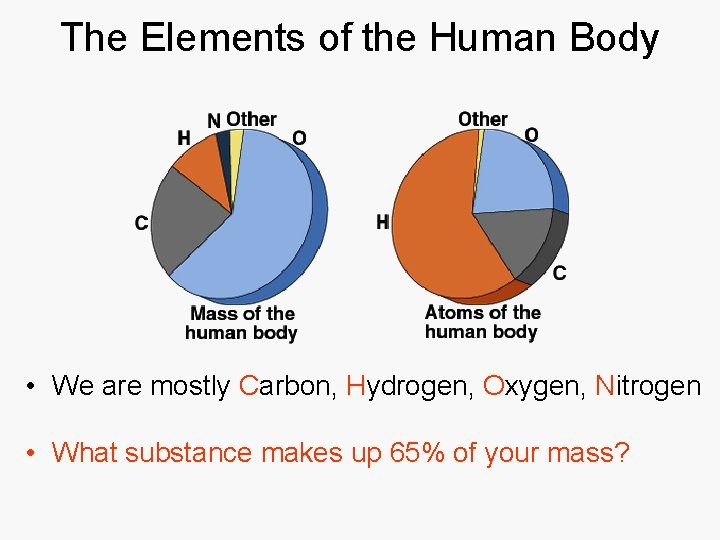
The Elements of the Human Body • We are mostly Carbon, Hydrogen, Oxygen, Nitrogen • What substance makes up 65% of your mass?

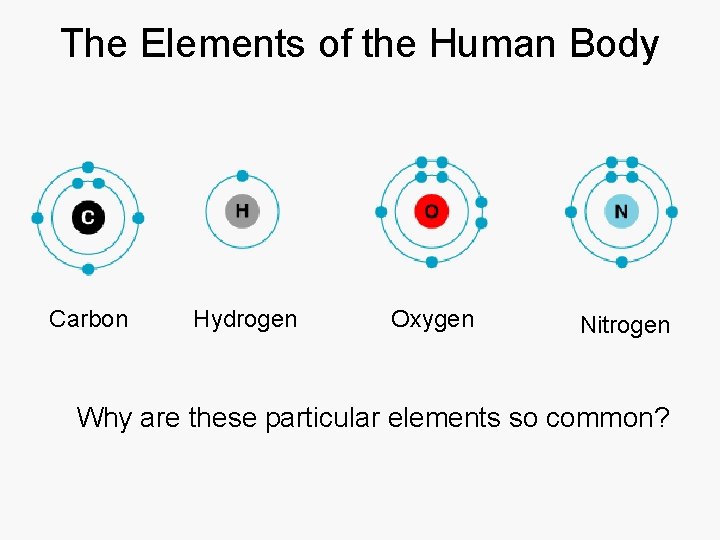
The Elements of the Human Body Carbon Hydrogen Oxygen Nitrogen Why are these particular elements so common?

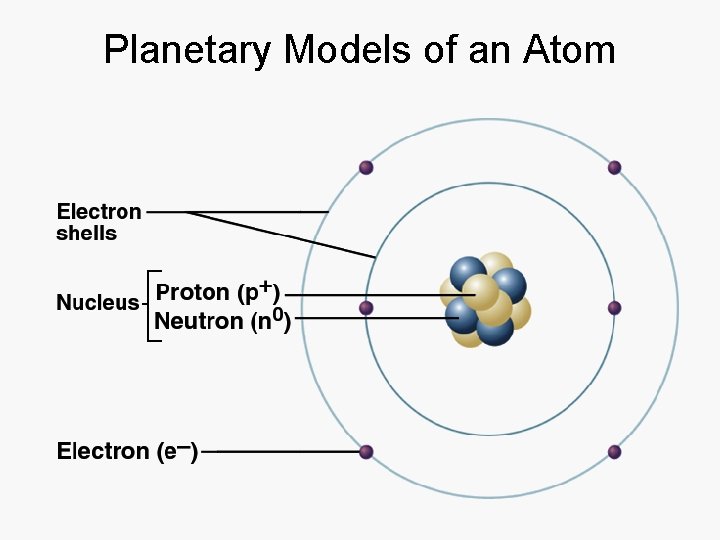
Planetary Models of an Atom

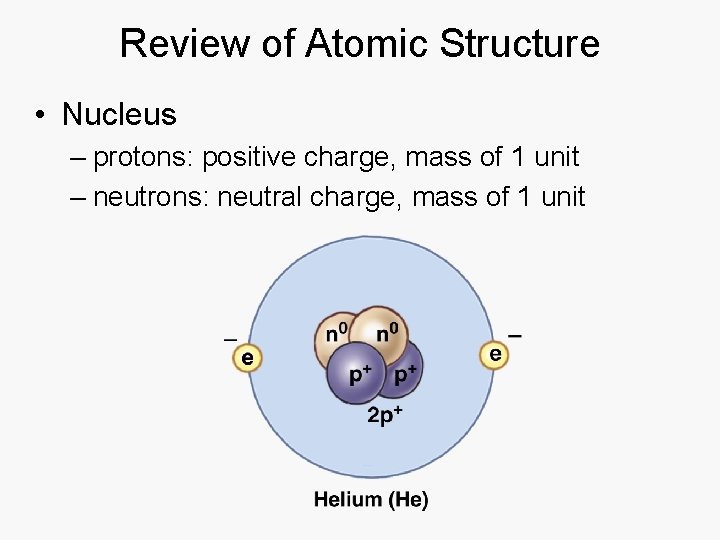
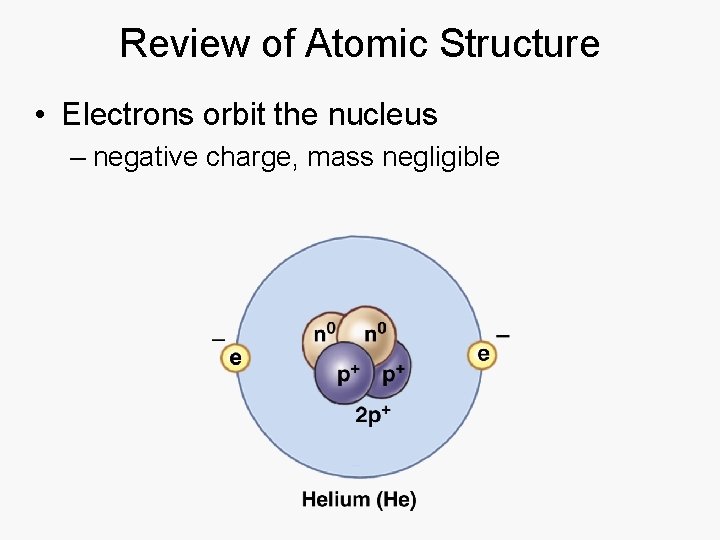
Review of Atomic Structure • Nucleus – protons: positive charge, mass of 1 unit – neutrons: neutral charge, mass of 1 unit

Review of Atomic Structure • Electrons orbit the nucleus – negative charge, mass negligible

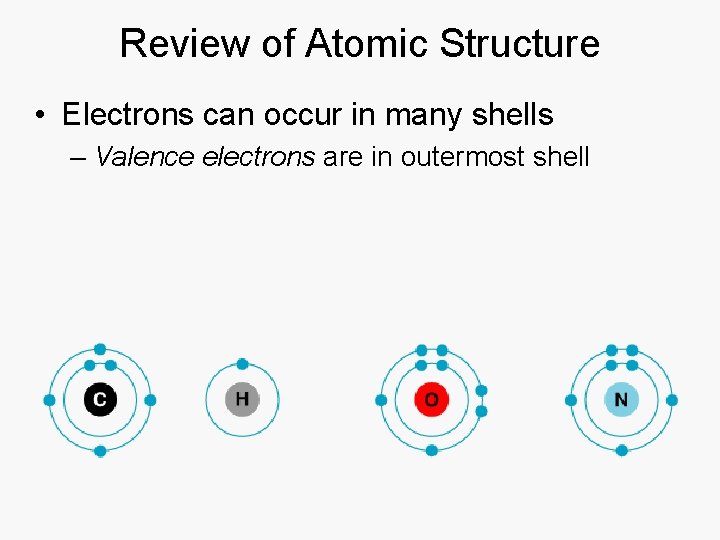
Review of Atomic Structure • Electrons can occur in many shells – Valence electrons are in outermost shell

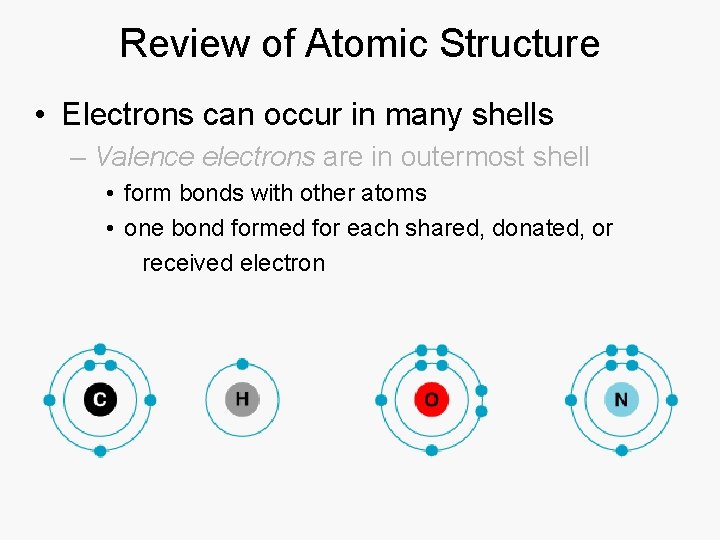
Review of Atomic Structure • Electrons can occur in many shells – Valence electrons are in outermost shell • form bonds with other atoms • one bond formed for each shared, donated, or received electron

What Determines the Number of Bonds? • The Duet Rule – Hydrogen wants 2 electrons in shell

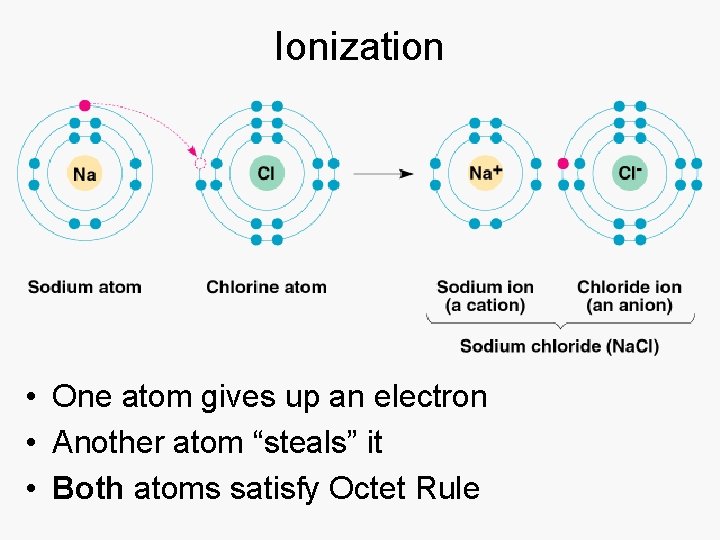
What Determines the Number of Bonds? • The Duet Rule – Hydrogen wants 2 electrons in shell • The Octet Rule – Atoms want to fill their outer shell – Shells 2 & 3 hold up to 8 electrons

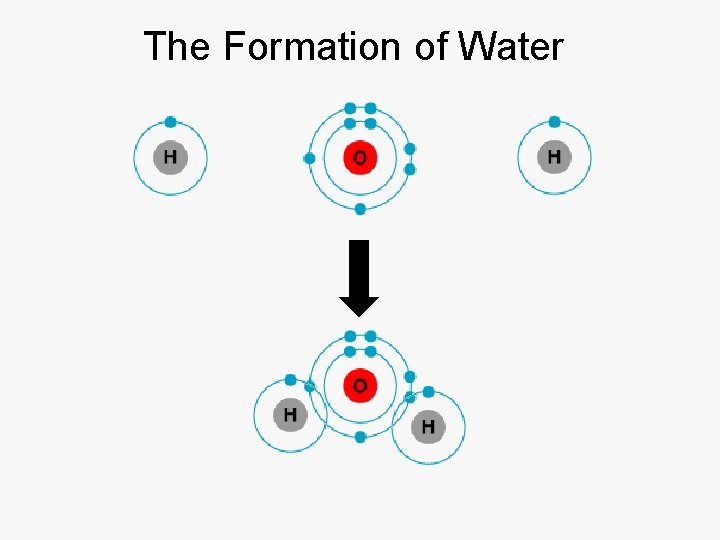
The Formation of Water

Chemistry of Life I. Properties of Atoms II. Chemical Bonds III. Reactions

Chemical Bonds • Covalent bonds • Hydrogen bonds • Ionic bonds

Covalent Bonds • Sharing of valence electrons • Types of covalent bonds 1. Single, double, or triple covalent bond • 2, 4, or 6 electrons are shared

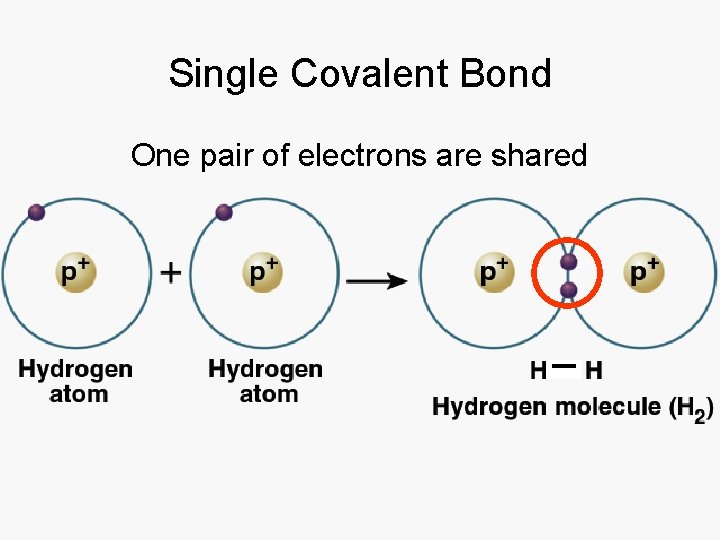
Single Covalent Bond One pair of electrons are shared

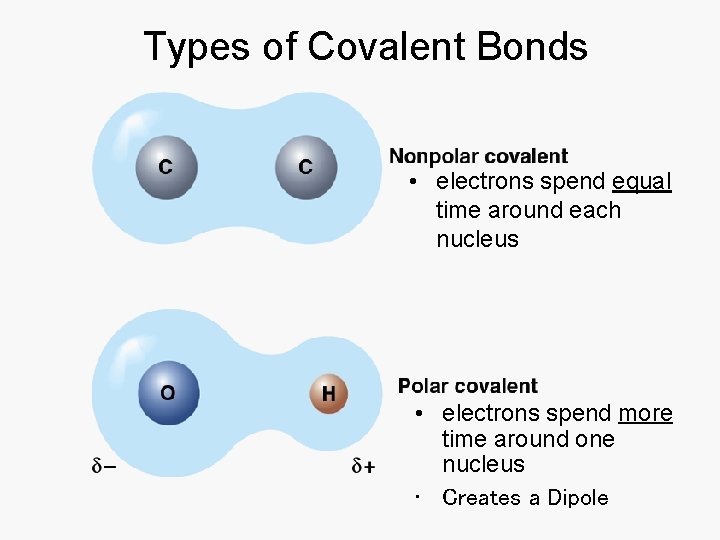
Covalent Bonds • • Sharing of valence electrons Types of covalent bonds 2. Nonpolar or polar covalent bond • Share electrons evenly or not

Types of Covalent Bonds • electrons spend equal time around each nucleus • electrons spend more time around one nucleus • Creates a Dipole

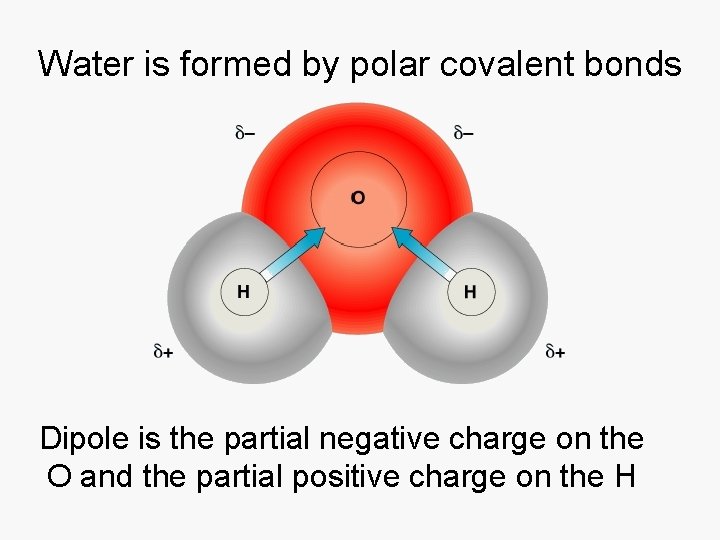
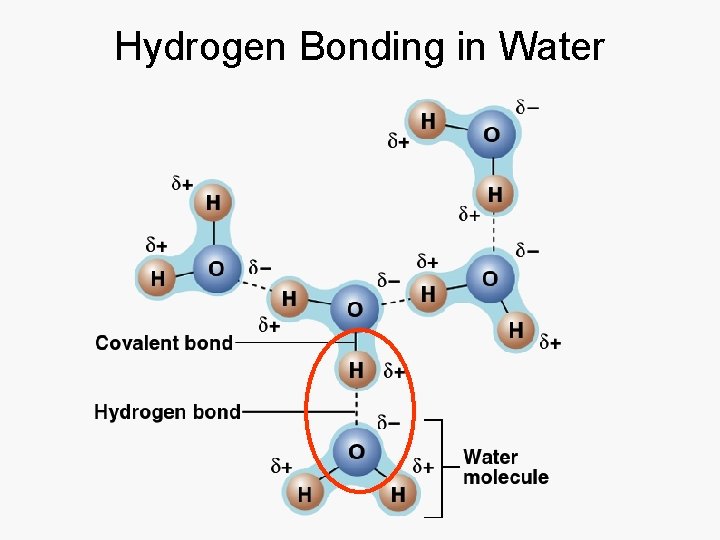
Water is formed by polar covalent bonds Dipole is the partial negative charge on the O and the partial positive charge on the H

Covalent Bonds are Really Strong Bond…… Covalent Bond

Chemical Bonds • Covalent bonds • Hydrogen bonds • Ionic bonds

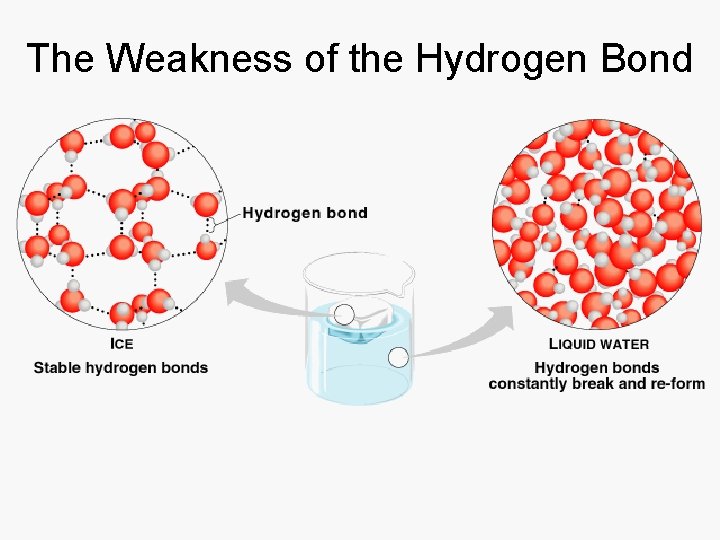
Hydrogen Bonds • Weakest of the bonds • Form because of the dipole • Greatest physiological importance – properties of water – shape of proteins and DNA

Hydrogen Bonding in Water

The Weakness of the Hydrogen Bond

Hydrogen Bonds Create Surface Tension It’s why a belly flop hurts… …and how insects walk on water

Chemical Bonds • Covalent bonds • Hydrogen bonds • Ionic bonds

Ionic Bonds • Attraction of charged atoms (ions) • Weak bonds that are readily broken

Ionization • One atom gives up an electron • Another atom “steals” it • Both atoms satisfy Octet Rule

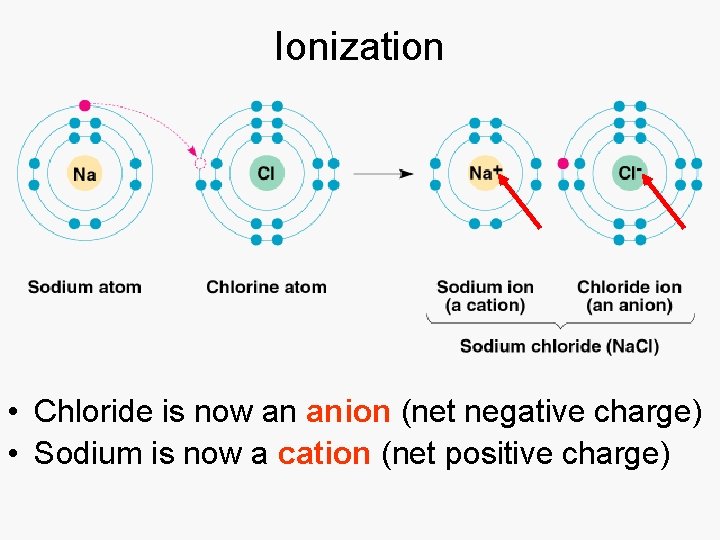
Ionization • Chloride is now an anion (net negative charge) • Sodium is now a cation (net positive charge)

Hydrogen & ionic bonds are like a Hollywood marriage: Weak Don’t Hassel the Hoff

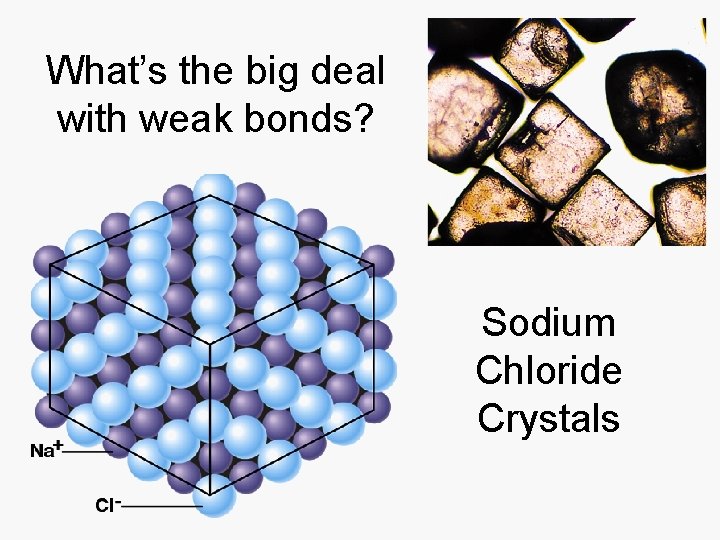
What’s the big deal with weak bonds? Sodium Chloride Crystals

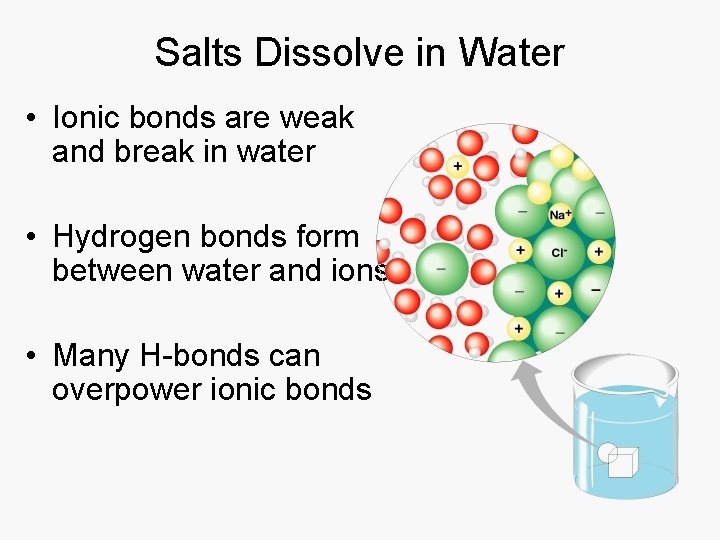
Salts Dissolve in Water • Ionic bonds are weak and break in water • Hydrogen bonds form between water and ions. • Many H-bonds can overpower ionic bonds

Chemistry of Life I. Properties of Atoms II. Chemical Bonds III. Reactions

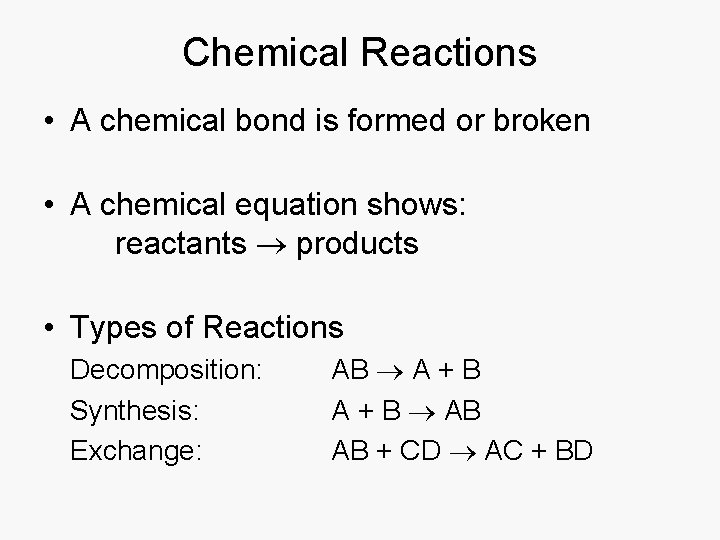
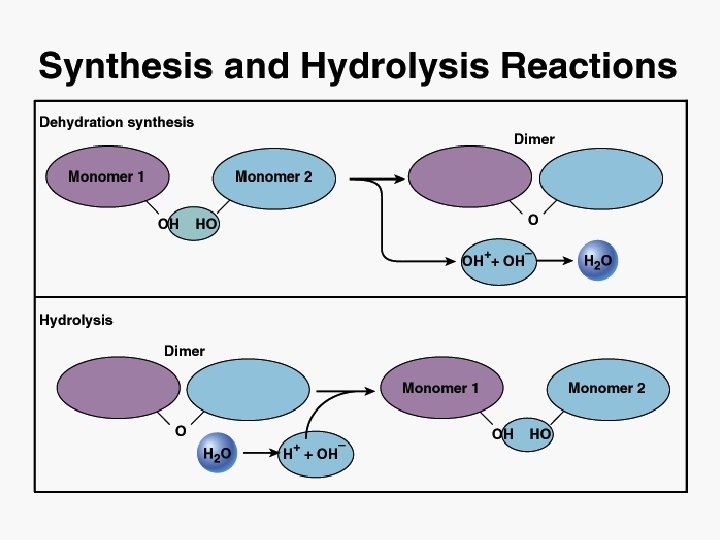
Chemical Reactions • A chemical bond is formed or broken • A chemical equation shows: reactants products • Types of Reactions Decomposition: Synthesis: Exchange: AB A + B AB AB + CD AC + BD


Summary • Life is composed mainly of Carbon, Hydrogen, Oxygen, and Nitrogen. Carbon serves as the structural backbone for most biological molecules. • An atom’s reactive properties are determined by its valence. • Atoms can form covalent, hydrogen, or ionic bonds. • Reactants can unite to form larger energy-rich molecules or decompose to form smaller energypoor molecules.