The 3 CPO Study Efficacy of Noninvasive Ventilation






- Slides: 30

The 3 CPO Study Efficacy of Non-invasive Ventilation in Patients with Acute Cardiogenic Pulmonary Oedema The 3 CPO Trial ISRCTN 07448447 David Newby University of Edinburgh, UK on behalf of the 3 CPO Investigators

The 3 CPO Study Declarations • Conflicts of Interest - None • This project was funded by the NIHR Health Technology Assessment Programme (project number 01/43/01). The views and opinions expressed herein are those of the investigators and do not necessarily reflect those of the Department of Health.

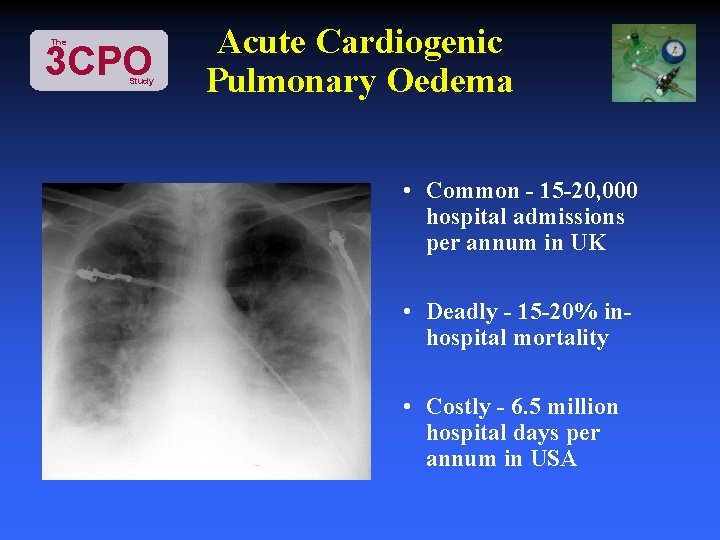
The 3 CPO Study Acute Cardiogenic Pulmonary Oedema • Common - 15 -20, 000 hospital admissions per annum in UK • Deadly - 15 -20% inhospital mortality • Costly - 6. 5 million hospital days per annum in USA

The 3 CPO Study Acute Cardiogenic Pulmonary Oedema • Loop Diuretic Therapy • Nitrate Therapy • Oxygen Therapy • (Opiates) • Treat Underlying Cause

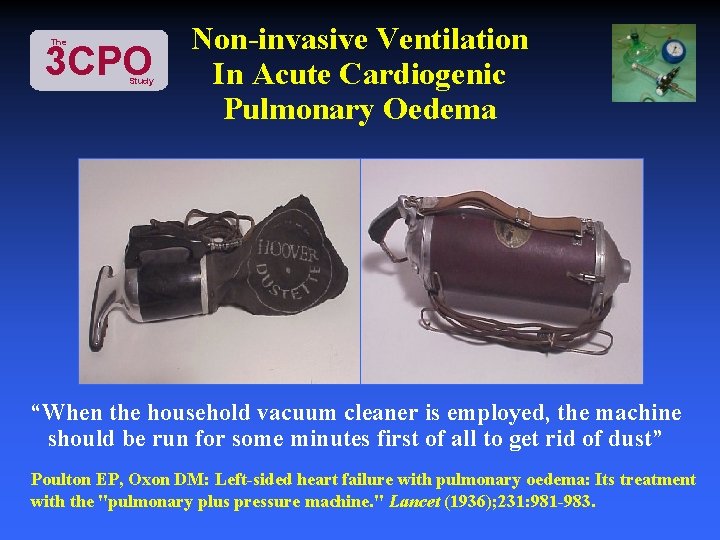
The 3 CPO Study Non-invasive Ventilation In Acute Cardiogenic Pulmonary Oedema “When the household vacuum cleaner is employed, the machine should be run for some minutes first of all to get rid of dust” Poulton EP, Oxon DM: Left-sided heart failure with pulmonary oedema: Its treatment with the "pulmonary plus pressure machine. " Lancet (1936); 231: 981 -983.

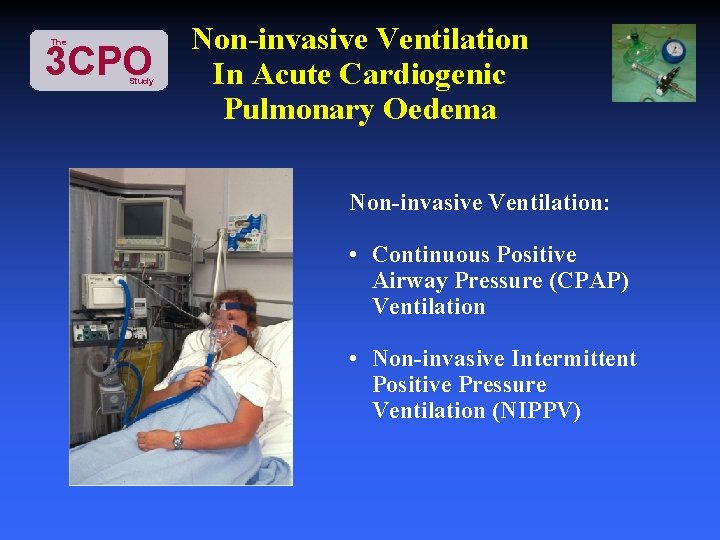
The 3 CPO Study Non-invasive Ventilation In Acute Cardiogenic Pulmonary Oedema Non-invasive Ventilation: • Continuous Positive Airway Pressure (CPAP) Ventilation • Non-invasive Intermittent Positive Pressure Ventilation (NIPPV)

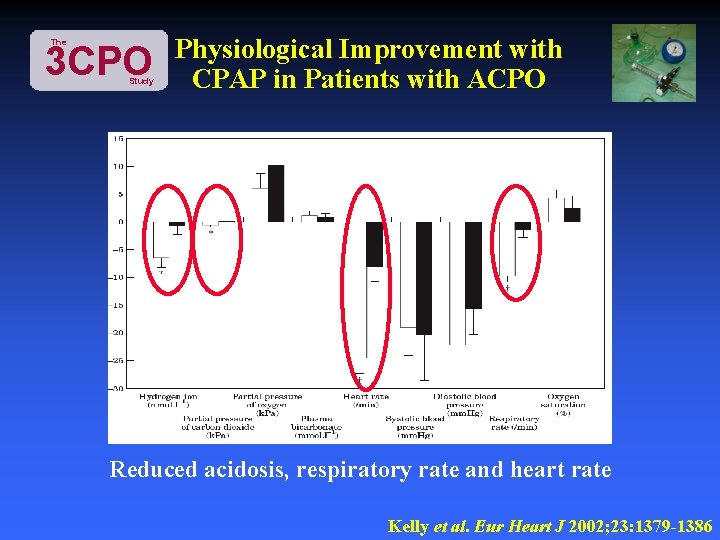
The 3 CPO Study Physiological Improvement with CPAP in Patients with ACPO Reduced acidosis, respiratory rate and heart rate Kelly et al. Eur Heart J 2002; 23: 1379 -1386

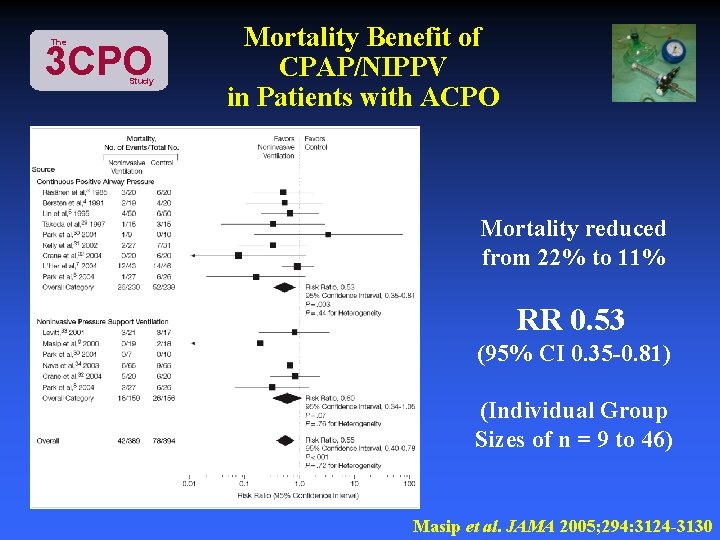
The 3 CPO Study Mortality Benefit of CPAP/NIPPV in Patients with ACPO Mortality reduced from 22% to 11% RR 0. 53 (95% CI 0. 35 -0. 81) (Individual Group Sizes of n = 9 to 46) Masip et al. JAMA 2005; 294: 3124 -3130

The 3 CPO Study Aims and Hypothesis In patients with acute cardiogenic pulmonary oedema: Aims • Clinical effectiveness of non-invasive ventilation • Comparative effectiveness of CPAP and NIPPV • Safety of non-invasive ventilation Hypothesis: • Non-invasive ventilation reduces mortality

The 3 CPO Study • • Trial Design Multicentre national randomised controlled trial Setting: Emergency Department Prospective ‘open-label’ trial Three treatment interventions in a 1: 1: 1 randomisation: ‘standard’ oxygen therapy, CPAP or NIPPV

The 3 CPO Study Inclusion Criteria Patients older than 16 years of age Signs and symptoms consistent with acute cardiogenic pulmonary oedema as the principal clinical complaint: acute dyspnoea and bilateral crackles on chest auscultation Chest radiograph confirming the diagnosis of acute cardiogenic pulmonary oedema: typical features of interstitial oedema present Arterial blood gas analysis with a p. H of <7. 35 (hydrogen ion concentration >45 nmol/L) Respiratory rate of >20 breaths per minute

The 3 CPO Study Exclusion Criteria Severely altered consciousness (unconscious or responding to pain only) Need for immediate lifesaving intervention Patients requiring thrombolysis or percutaneous coronary intervention for acute ST segment elevation myocardial infarction A clear alternative primary diagnosis An inability to provide informed consent at any time within the trial period Previous inclusion in the 3 CPO study

The 3 CPO Study Consent • Written informed consent • If unable to give written consent: – witnessed verbal consent – relatives’ assent is obtained. Verbal patient consent is witnessed in writing by a second individual involved in the patient’s clinical care. Subsequent written consent is obtained as soon as possible prior to the patient’s data being used in the trial and normally within one week of recruitment. MREC/02/0/74

The 3 CPO Study Intervention • Randomised (1: 1: 1) to: – Standard oxygen therapy (by facial mask) – CPAP (5 cm. H 2 O up titrated to a maximum of 15 cm. H 2 O) – NIPPV (8/4 cm. H 2 O up titrated to a maximum of 20/10 cm. H 2 O) • Inhaled oxygen of 60% • Attending physicians were encouraged to use nitrate and diuretic therapy • Opiate therapy was administered at the discretion of the treating physician

The 3 CPO Study Power Calculation Randomisation of 400 patients per group. Intention-to-treat analysis. Co-primary end-point of mortality between standard oxygen therapy and non-invasive ventilation (CPAP or NIPPV). • 80% Power at P<0. 05, to detect a difference of 6% in mortality assuming a mortality of 15%. Co-primary end-point of mortality or intubation between CPAP and NIPPV • 80% Power at P<0. 05, to detect a difference of 7% in mortality and intubation rate assuming an event rate of 18%.

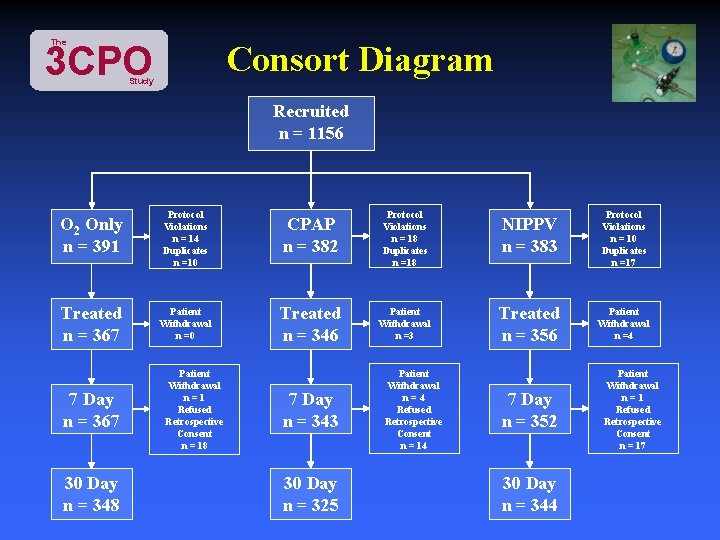
The 3 CPO Consort Diagram Study Recruited n = 1156 O 2 Only n = 391 Protocol Violations n = 14 Duplicates n =10 CPAP n = 382 Protocol Violations n = 18 Duplicates n =18 NIPPV n = 383 Protocol Violations n = 10 Duplicates n =17 Treated n = 367 Patient Withdrawal n =0 Treated n = 346 Patient Withdrawal n =3 Treated n = 356 Patient Withdrawal n =4 7 Day n = 367 30 Day n = 348 Patient Withdrawal n=1 Refused Retrospective Consent n = 18 7 Day n = 343 30 Day n = 325 Patient Withdrawal n=4 Refused Retrospective Consent n = 14 7 Day n = 352 30 Day n = 344 Patient Withdrawal n=1 Refused Retrospective Consent n = 17

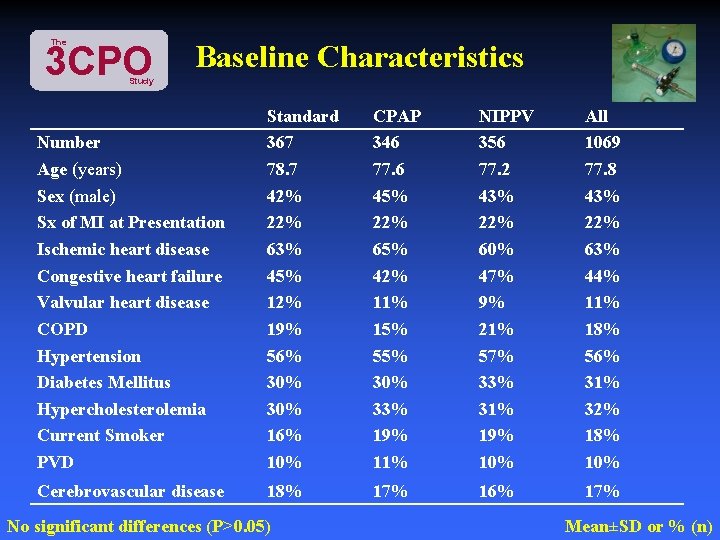
The 3 CPO Baseline Characteristics Study Number Age (years) Sex (male) Sx of MI at Presentation Ischemic heart disease Congestive heart failure Valvular heart disease COPD Hypertension Diabetes Mellitus Hypercholesterolemia Current Smoker PVD Standard 367 78. 7 42% 22% 63% 45% 12% 19% 56% 30% 16% 10% CPAP 346 77. 6 45% 22% 65% 42% 11% 15% 55% 30% 33% 19% 11% NIPPV 356 77. 2 43% 22% 60% 47% 9% 21% 57% 33% 31% 19% 10% All 1069 77. 8 43% 22% 63% 44% 11% 18% 56% 31% 32% 18% 10% Cerebrovascular disease 18% 17% 16% 17% No significant differences (P>0. 05) Mean±SD or % (n)

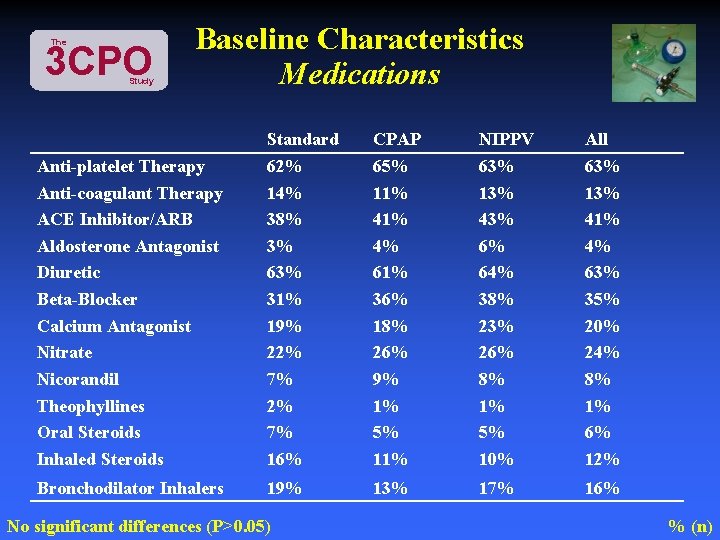
The 3 CPO Study Baseline Characteristics Medications Anti-platelet Therapy Anti-coagulant Therapy ACE Inhibitor/ARB Aldosterone Antagonist Diuretic Beta-Blocker Calcium Antagonist Nitrate Nicorandil Theophyllines Oral Steroids Inhaled Steroids Standard 62% 14% 38% 3% 63% 31% 19% 22% 7% 16% CPAP 65% 11% 4% 61% 36% 18% 26% 9% 1% 5% 11% NIPPV 63% 13% 43% 6% 64% 38% 23% 26% 8% 1% 5% 10% All 63% 13% 41% 4% 63% 35% 20% 24% 8% 1% 6% 12% Bronchodilator Inhalers 19% 13% 17% 16% No significant differences (P>0. 05) % (n)

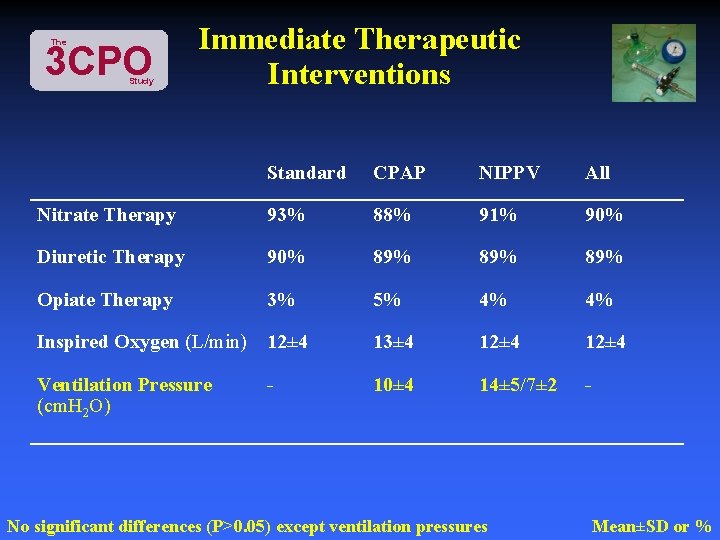
The 3 CPO Study Immediate Therapeutic Interventions Standard CPAP NIPPV All Nitrate Therapy 93% 88% 91% 90% Diuretic Therapy 90% 89% 89% Opiate Therapy 3% 5% 4% 4% Inspired Oxygen (L/min) 12± 4 13± 4 12± 4 Ventilation Pressure (cm. H 2 O) 10± 4 14± 5/7± 2 - - No significant differences (P>0. 05) except ventilation pressures Mean±SD or %

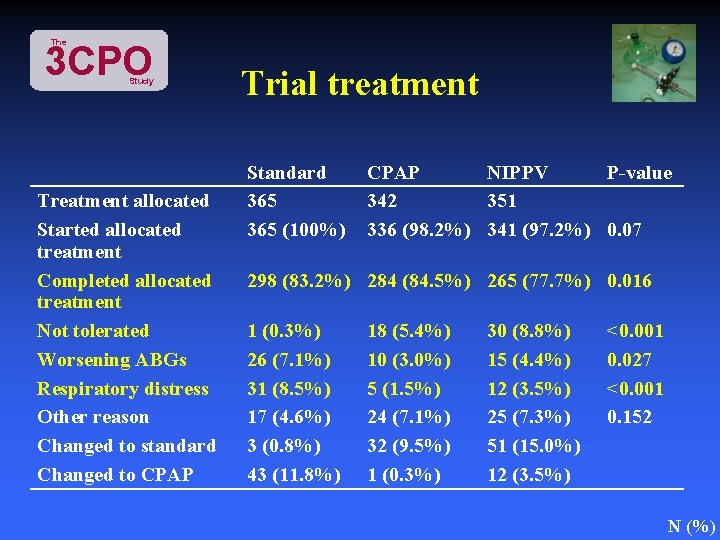
The 3 CPO Study Treatment allocated Started allocated treatment Completed allocated treatment Not tolerated Worsening ABGs Respiratory distress Other reason Changed to standard Changed to CPAP Trial treatment Standard 365 (100%) CPAP NIPPV P-value 342 351 336 (98. 2%) 341 (97. 2%) 0. 07 298 (83. 2%) 284 (84. 5%) 265 (77. 7%) 0. 016 1 (0. 3%) 26 (7. 1%) 31 (8. 5%) 17 (4. 6%) 3 (0. 8%) 43 (11. 8%) 18 (5. 4%) 10 (3. 0%) 5 (1. 5%) 24 (7. 1%) 32 (9. 5%) 1 (0. 3%) 30 (8. 8%) 15 (4. 4%) 12 (3. 5%) 25 (7. 3%) 51 (15. 0%) 12 (3. 5%) <0. 001 0. 027 <0. 001 0. 152 N (%)

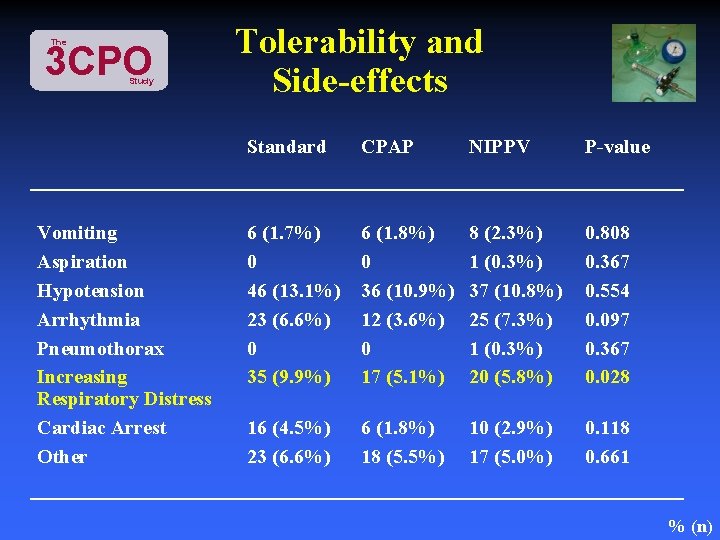
The 3 CPO Study Vomiting Aspiration Hypotension Arrhythmia Pneumothorax Increasing Respiratory Distress Cardiac Arrest Other Tolerability and Side-effects Standard CPAP NIPPV P-value 6 (1. 7%) 0 46 (13. 1%) 23 (6. 6%) 0 35 (9. 9%) 6 (1. 8%) 0 36 (10. 9%) 12 (3. 6%) 0 17 (5. 1%) 8 (2. 3%) 1 (0. 3%) 37 (10. 8%) 25 (7. 3%) 1 (0. 3%) 20 (5. 8%) 0. 808 0. 367 0. 554 0. 097 0. 367 0. 028 16 (4. 5%) 23 (6. 6%) 6 (1. 8%) 18 (5. 5%) 10 (2. 9%) 17 (5. 0%) 0. 118 0. 661 % (n)

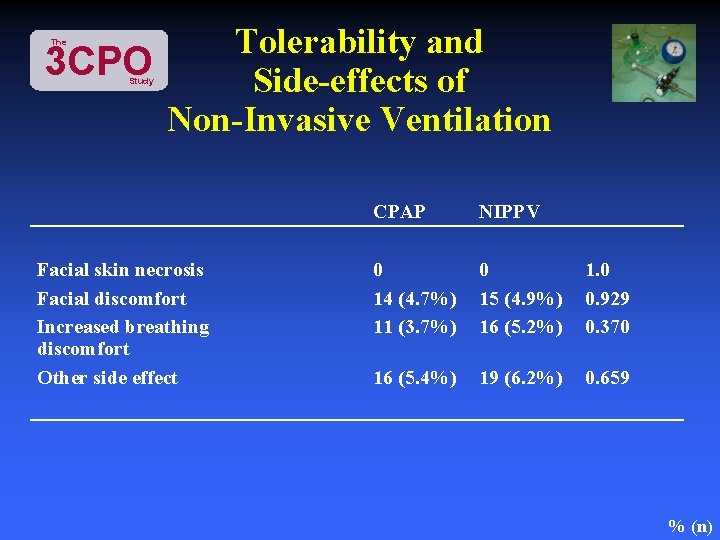
Tolerability and 3 CPO Side-effects of Non-Invasive Ventilation The Study Facial skin necrosis Facial discomfort Increased breathing discomfort Other side effect CPAP NIPPV 0 14 (4. 7%) 11 (3. 7%) 0 15 (4. 9%) 16 (5. 2%) 1. 0 0. 929 0. 370 16 (5. 4%) 19 (6. 2%) 0. 659 % (n)

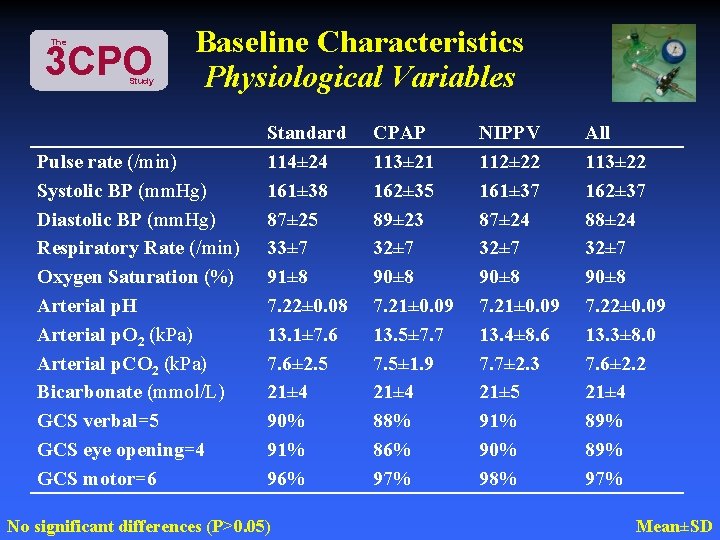
The 3 CPO Study Baseline Characteristics Physiological Variables Pulse rate (/min) Systolic BP (mm. Hg) Diastolic BP (mm. Hg) Respiratory Rate (/min) Oxygen Saturation (%) Arterial p. H Arterial p. O 2 (k. Pa) Arterial p. CO 2 (k. Pa) Bicarbonate (mmol/L) GCS verbal=5 GCS eye opening=4 GCS motor=6 Standard 114± 24 161± 38 87± 25 33± 7 91± 8 7. 22± 0. 08 13. 1± 7. 6± 2. 5 21± 4 90% 91% 96% No significant differences (P>0. 05) CPAP 113± 21 162± 35 89± 23 32± 7 90± 8 7. 21± 0. 09 13. 5± 7. 7 7. 5± 1. 9 21± 4 88% 86% 97% NIPPV 112± 22 161± 37 87± 24 32± 7 90± 8 7. 21± 0. 09 13. 4± 8. 6 7. 7± 2. 3 21± 5 91% 90% 98% All 113± 22 162± 37 88± 24 32± 7 90± 8 7. 22± 0. 09 13. 3± 8. 0 7. 6± 2. 2 21± 4 89% 97% Mean±SD

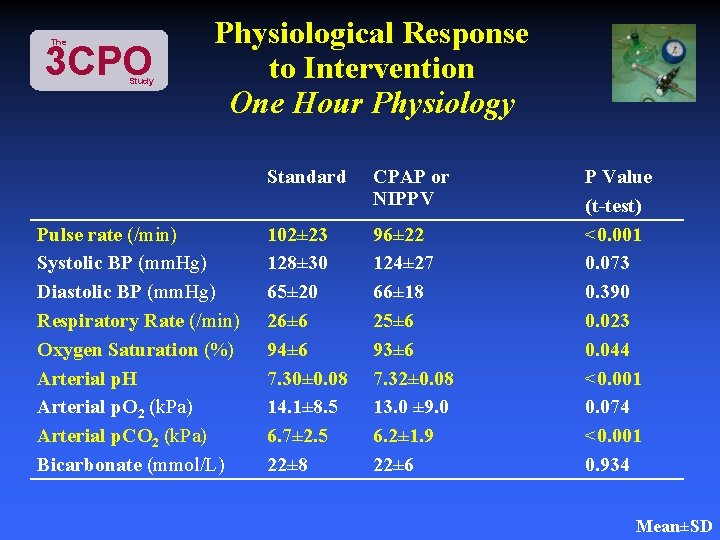
The 3 CPO Study Physiological Response to Intervention One Hour Physiology Pulse rate (/min) Systolic BP (mm. Hg) Diastolic BP (mm. Hg) Respiratory Rate (/min) Oxygen Saturation (%) Arterial p. H Arterial p. O 2 (k. Pa) Arterial p. CO 2 (k. Pa) Bicarbonate (mmol/L) Standard CPAP or NIPPV 102± 23 128± 30 65± 20 26± 6 94± 6 7. 30± 0. 08 14. 1± 8. 5 6. 7± 2. 5 22± 8 96± 22 124± 27 66± 18 25± 6 93± 6 7. 32± 0. 08 13. 0 ± 9. 0 6. 2± 1. 9 22± 6 P Value (t-test) <0. 001 0. 073 0. 390 0. 023 0. 044 <0. 001 0. 074 <0. 001 0. 934 Mean±SD

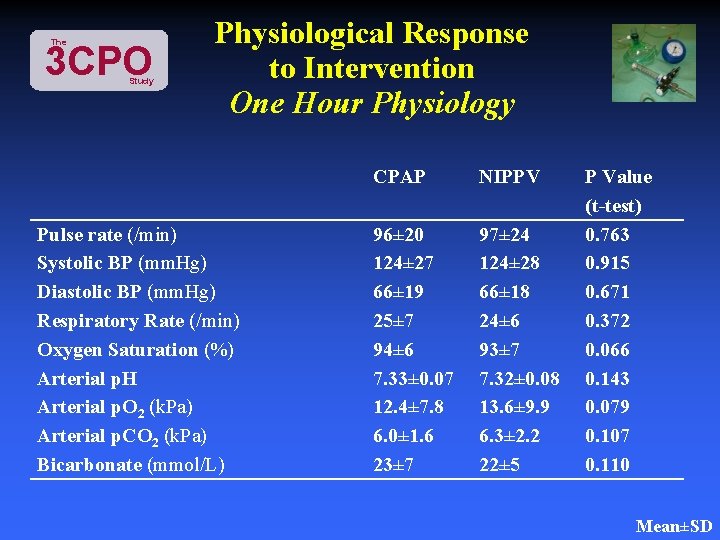
The 3 CPO Study Physiological Response to Intervention One Hour Physiology Pulse rate (/min) Systolic BP (mm. Hg) Diastolic BP (mm. Hg) Respiratory Rate (/min) Oxygen Saturation (%) Arterial p. H Arterial p. O 2 (k. Pa) Arterial p. CO 2 (k. Pa) Bicarbonate (mmol/L) CPAP NIPPV 96± 20 124± 27 66± 19 25± 7 94± 6 7. 33± 0. 07 12. 4± 7. 8 6. 0± 1. 6 23± 7 97± 24 124± 28 66± 18 24± 6 93± 7 7. 32± 0. 08 13. 6± 9. 9 6. 3± 2. 2 22± 5 P Value (t-test) 0. 763 0. 915 0. 671 0. 372 0. 066 0. 143 0. 079 0. 107 0. 110 Mean±SD

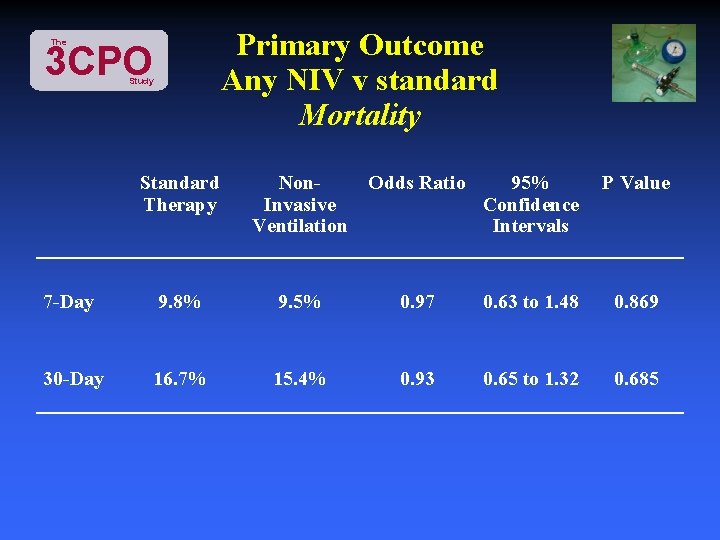
Primary Outcome Any NIV v standard Mortality The 3 CPO Study Standard Therapy Non. Invasive Ventilation Odds Ratio 95% Confidence Intervals P Value 7 -Day 9. 8% 9. 5% 0. 97 0. 63 to 1. 48 0. 869 30 -Day 16. 7% 15. 4% 0. 93 0. 65 to 1. 32 0. 685

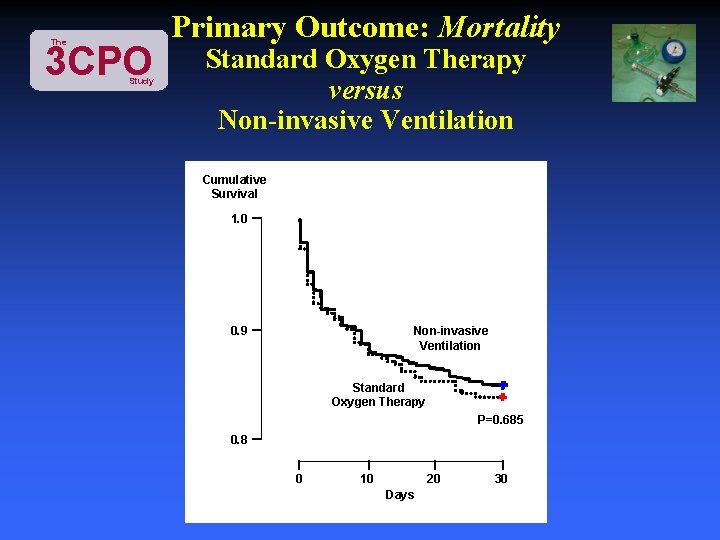
The 3 CPO Study Primary Outcome: Mortality Standard Oxygen Therapy versus Non-invasive Ventilation Cumulative Survival 1. 0 0. 9 Non-invasive Ventilation Standard Oxygen Therapy P=0. 685 0. 8 0 10 20 Days 30

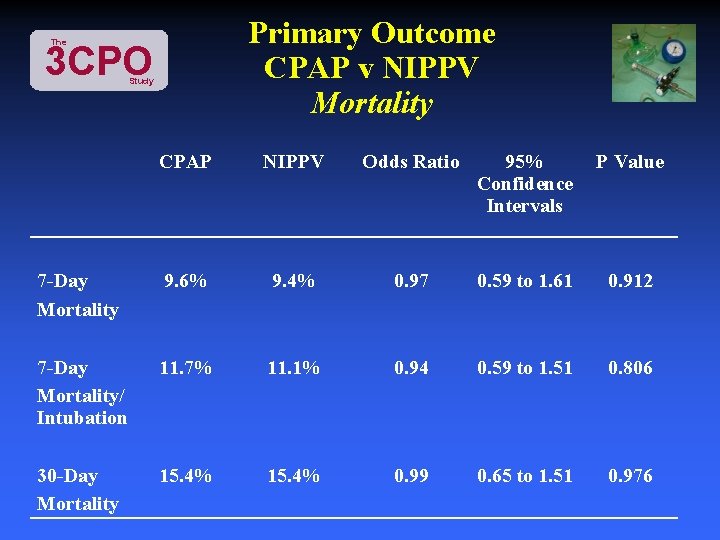
Primary Outcome CPAP v NIPPV Mortality The 3 CPO Study CPAP NIPPV Odds Ratio 95% Confidence Intervals P Value 7 -Day Mortality 9. 6% 9. 4% 0. 97 0. 59 to 1. 61 0. 912 7 -Day Mortality/ Intubation 11. 7% 11. 1% 0. 94 0. 59 to 1. 51 0. 806 30 -Day Mortality 15. 4% 0. 99 0. 65 to 1. 51 0. 976

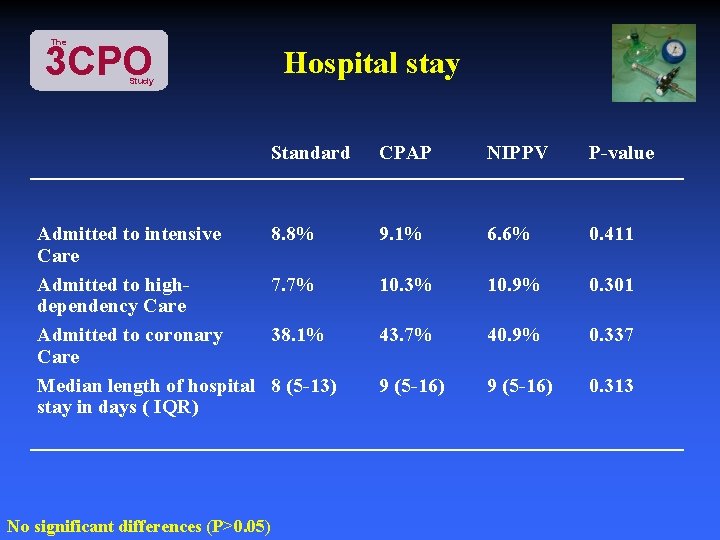
The 3 CPO Hospital stay Study Admitted to intensive Care Admitted to highdependency Care Admitted to coronary Care Median length of hospital stay in days ( IQR) Standard CPAP NIPPV P-value 8. 8% 9. 1% 6. 6% 0. 411 7. 7% 10. 3% 10. 9% 0. 301 38. 1% 43. 7% 40. 9% 0. 337 8 (5 -13) 9 (5 -16) 0. 313 No significant differences (P>0. 05)

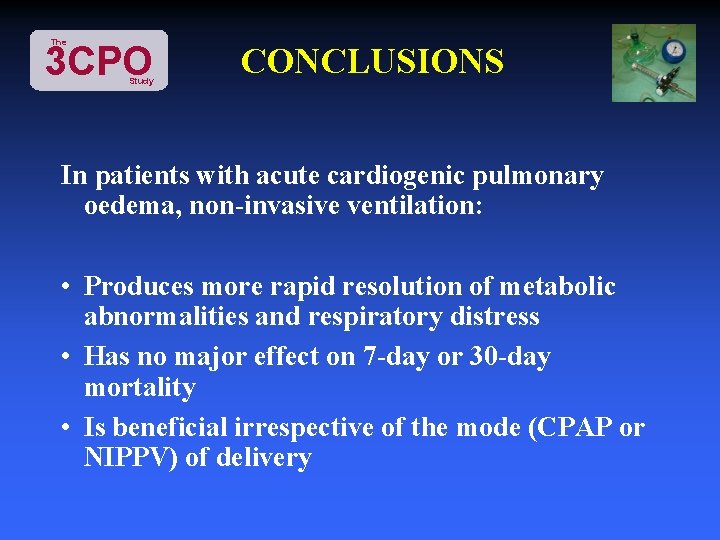
The 3 CPO Study CONCLUSIONS In patients with acute cardiogenic pulmonary oedema, non-invasive ventilation: • Produces more rapid resolution of metabolic abnormalities and respiratory distress • Has no major effect on 7 -day or 30 -day mortality • Is beneficial irrespective of the mode (CPAP or NIPPV) of delivery
 Non invasive cgm
Non invasive cgm Mode of ventilation
Mode of ventilation Cpo rising
Cpo rising Www.cpo.ucla.edu/holidaybox
Www.cpo.ucla.edu/holidaybox Insolventieverklaring
Insolventieverklaring Daiva vaitkevičiūtė
Daiva vaitkevičiūtė Navy officer fitrep examples
Navy officer fitrep examples Cpo precepts
Cpo precepts Niada cpo
Niada cpo Cpo respiratory
Cpo respiratory Navy cpo final night
Navy cpo final night What is role efficacy
What is role efficacy Drug efficacy
Drug efficacy Christina mellon
Christina mellon Efficacy potency
Efficacy potency Albert bandura self efficacy
Albert bandura self efficacy Potency vs efficacy
Potency vs efficacy Luminous efficacy comparison chart
Luminous efficacy comparison chart Collective teacher efficacy
Collective teacher efficacy Vaccine efficacy
Vaccine efficacy Nebido efficacy
Nebido efficacy Potency vs efficacy
Potency vs efficacy Drug efficacy
Drug efficacy Collective teacher efficacy
Collective teacher efficacy Types of personality in organisational behaviour
Types of personality in organisational behaviour Vaccine efficacy
Vaccine efficacy Sources of self efficacy
Sources of self efficacy Slidetodoc.com
Slidetodoc.com Collective teacher efficacy
Collective teacher efficacy Tư thế worm breton
Tư thế worm breton ưu thế lai là gì
ưu thế lai là gì