Terms Energy Heat Calorie Joule Specific heat Calorimeter











- Slides: 32

Terms • Energy • Heat • Calorie • Joule • Specific heat • Calorimeter • Thermochemistry

Chapter 16 Thermochemistry

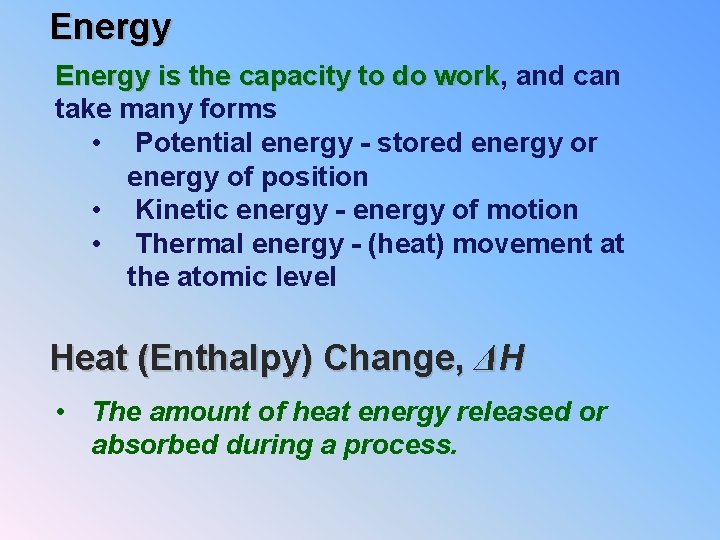
Energy is the capacity to do work, work and can take many forms • Potential energy - stored energy or energy of position • Kinetic energy - energy of motion • Thermal energy - (heat) movement at the atomic level Heat (Enthalpy) Change, ΔH • The amount of heat energy released or absorbed during a process.

Law of conservation of energy • Energy can be converted from one form to another, but neither created nor destroyed.

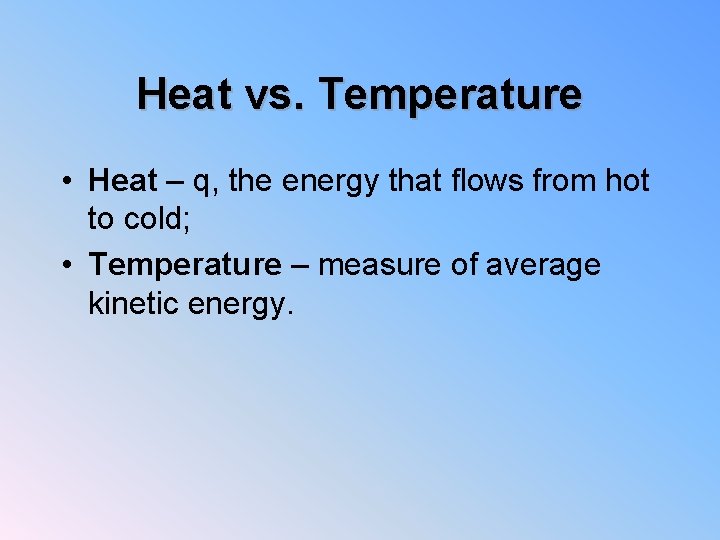
Heat vs. Temperature • Heat – q, the energy that flows from hot to cold; • Temperature – measure of average kinetic energy.

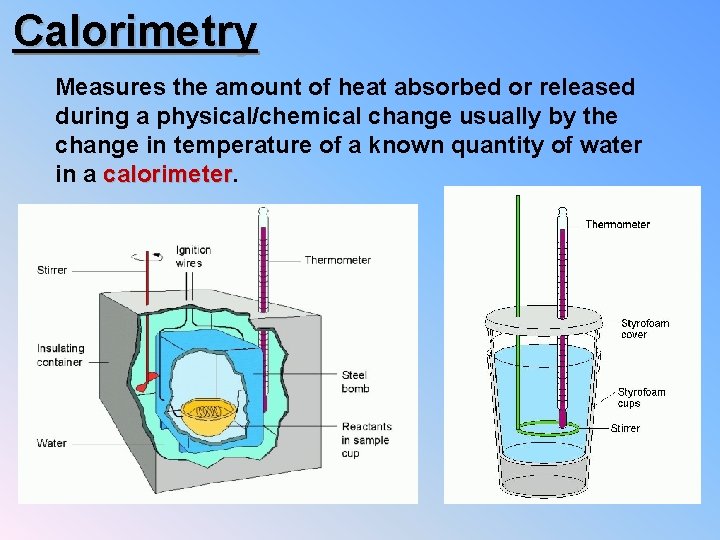
Calorimetry Measures the amount of heat absorbed or released during a physical/chemical change usually by the change in temperature of a known quantity of water in a calorimeter

Measuring heat • Metric system: calorie (cal) – Heat required to raise the temperature of 1 gram of pure water 1ºC • Food Calories differ from heat calories – 1 Calorie = 1000 cal • SI unit: joule (J) – 1 J = 0. 2390 cal – 1 cal = 4. 184 J

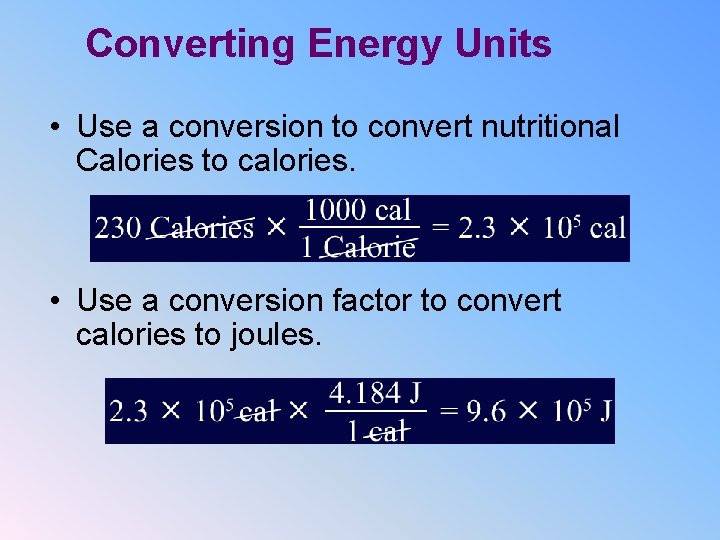
Converting Energy Units • Use a conversion to convert nutritional Calories to calories. • Use a conversion factor to convert calories to joules.

Practice • Convert the following – 934 Calories to calories – 7. 42 x 106 calories to Calories – 236 Calories to joules – 2. 59 x 106 calories to joules – 84 joules to calories – 7. 19 x 106 joules to Calories

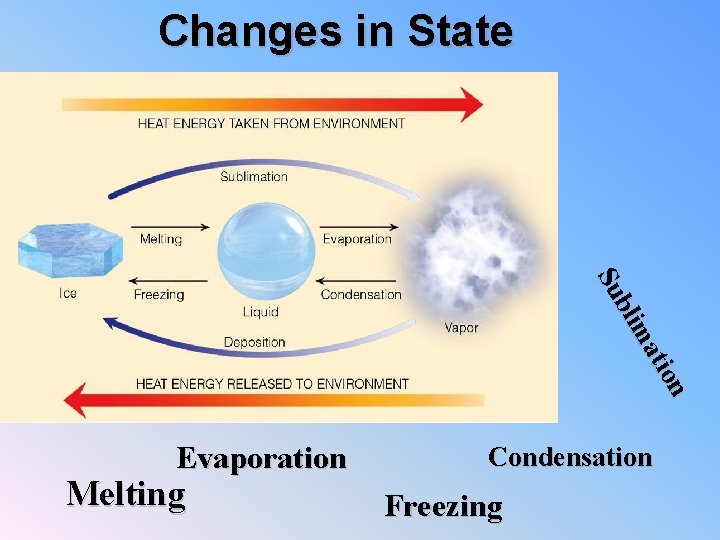
Changes in State n tio ma bli Su Evaporation Melting Condensation Freezing

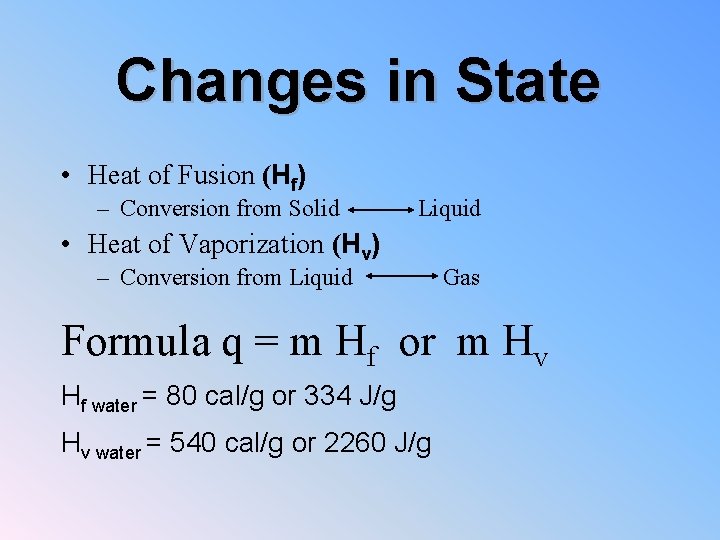
Changes in State • Heat of Fusion (Hf) – Conversion from Solid Liquid • Heat of Vaporization (Hv) – Conversion from Liquid Gas Formula q = m Hf or m Hv Hf water = 80 cal/g or 334 J/g Hv water = 540 cal/g or 2260 J/g

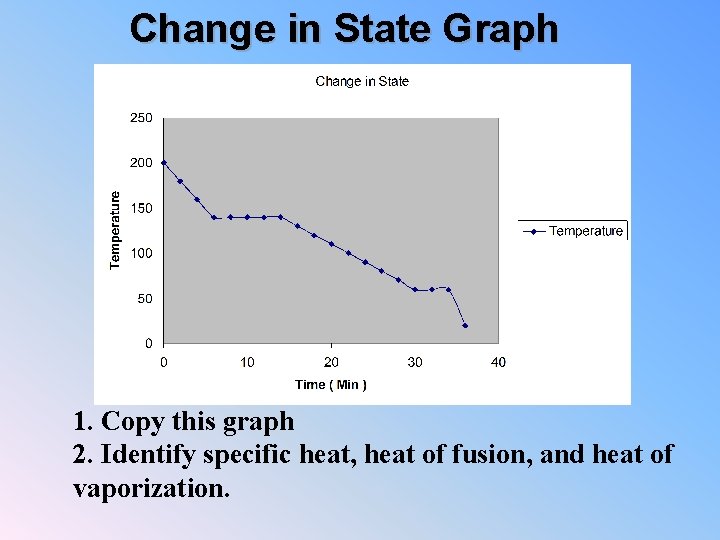
Change in State Graph 1. Copy this graph 2. Identify specific heat, heat of fusion, and heat of vaporization.

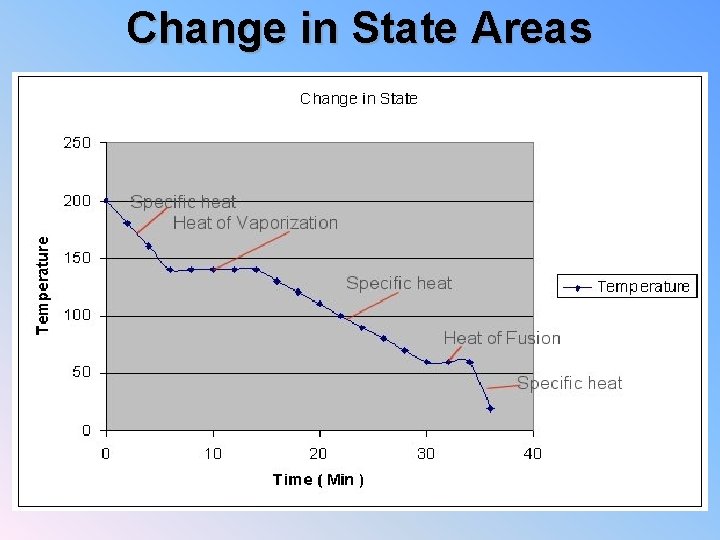
Change in State Areas

Sample Heat of Fusion • How much heat energy is needed to melt 5. 0 kilograms of water at its melting point? Q = m Hf = 5 kg x 334 J/kg = 1, 670 J Q = m Hf = 5000 g x 80 cal/g = 400, 000 cal

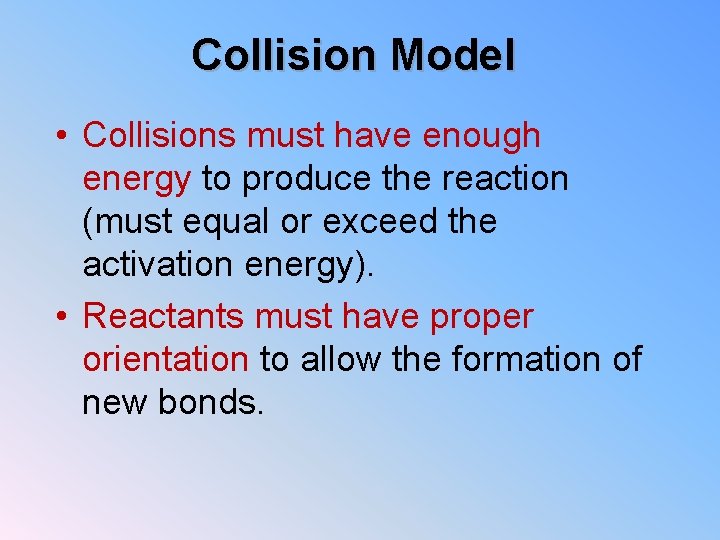
Collision Model • Collisions must have enough energy to produce the reaction (must equal or exceed the activation energy). • Reactants must have proper orientation to allow the formation of new bonds.

Activation Energy • The minimum energy required to transform reactants into the activated complex • Flame, spark, high temperature, radiation are all sources of activation energy

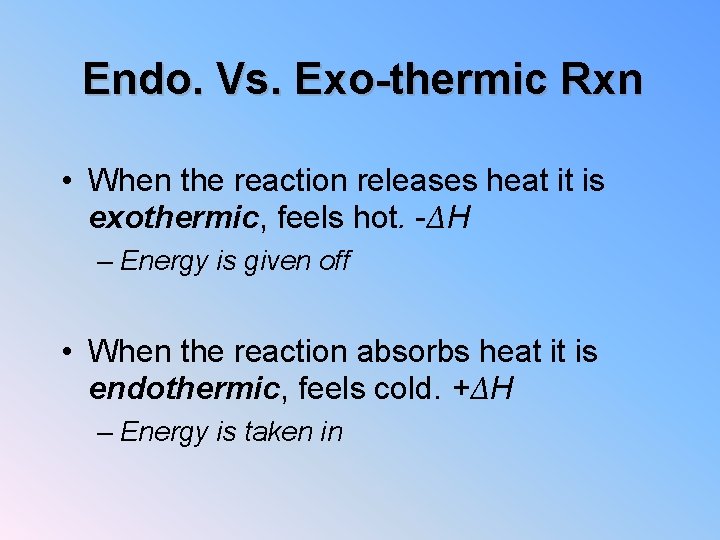
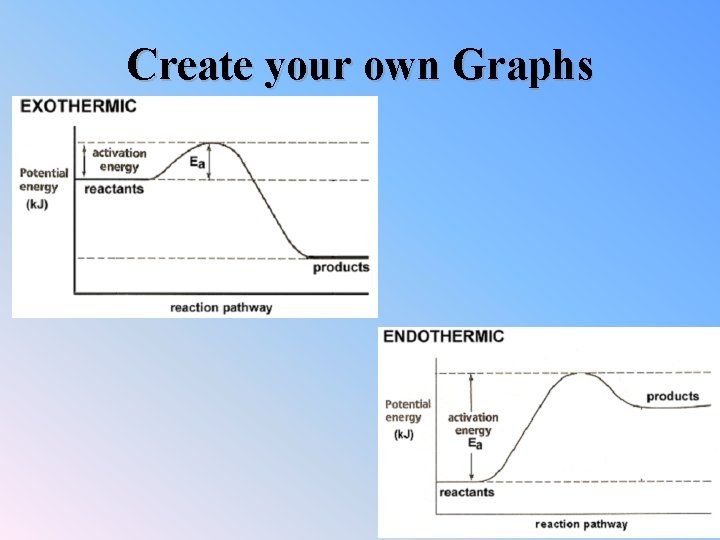
Endo. Vs. Exo-thermic Rxn • When the reaction releases heat it is exothermic, feels hot. -ΔH – Energy is given off • When the reaction absorbs heat it is endothermic, feels cold. +ΔH – Energy is taken in

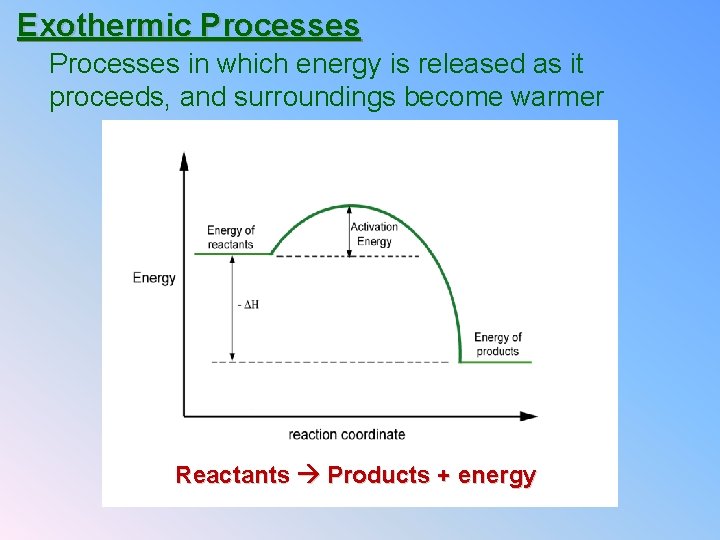
Exothermic Processes in which energy is released as it proceeds, and surroundings become warmer Reactants Products + energy

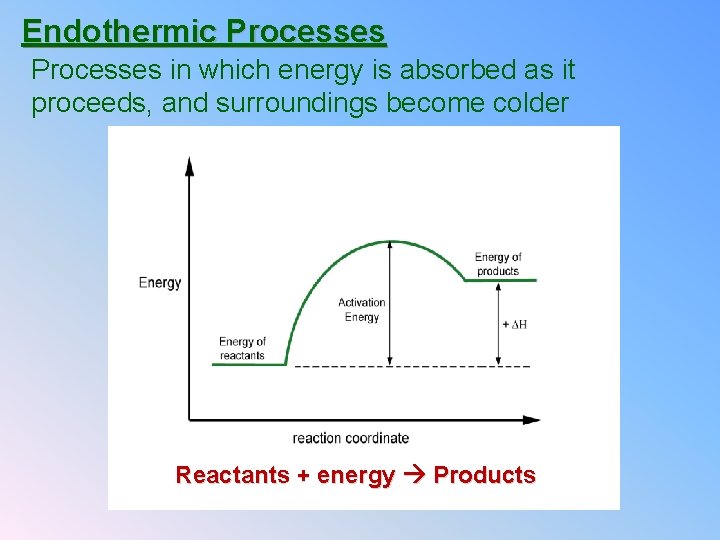
Endothermic Processes in which energy is absorbed as it proceeds, and surroundings become colder Reactants + energy Products

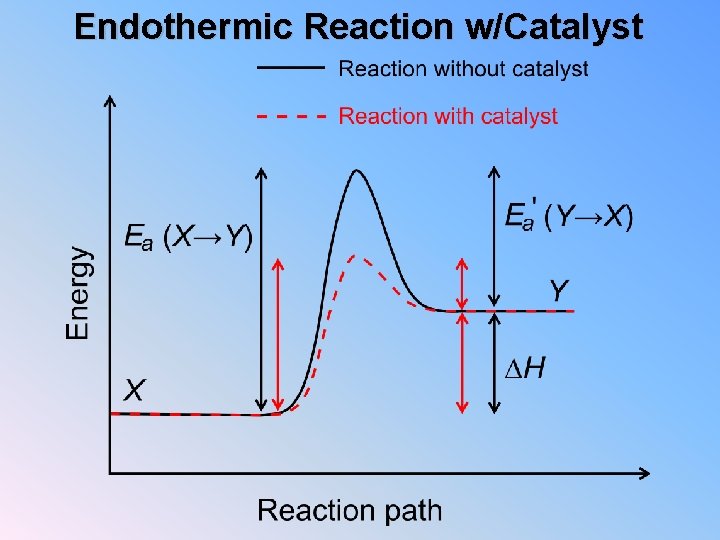
Endothermic Reaction w/Catalyst

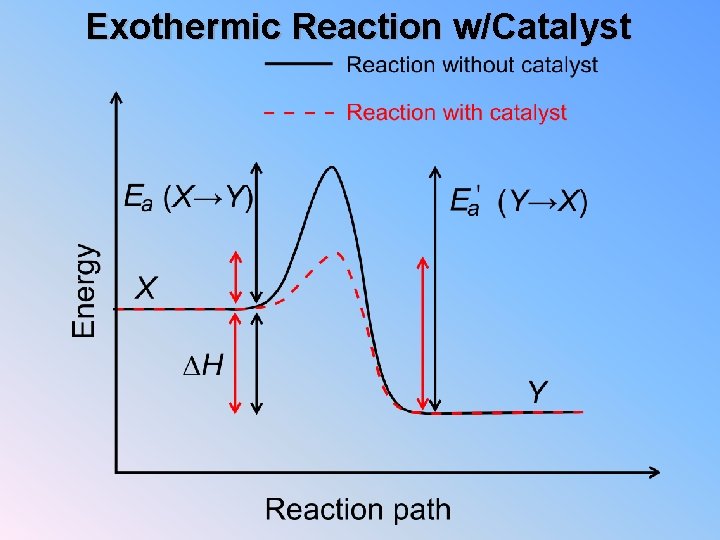
Exothermic Reaction w/Catalyst

Exo vs. Endo Exothermic 1. Which has more energy, reactants or products? 2. If the reaction ran backwards, which would have more energy, reactants or products? Endothermic 1. Which has more energy, reactants or products? 2. If the reaction ran backwards, which would have more energy, reactants or products? 3. Looking at your two diagrams, which has larger activation energy? 4. In general does an exothermic reaction have a larger activation energy running forwards or backwards? 5. In general does an endothermic reaction have a larger activation energy running forwards or backwards?

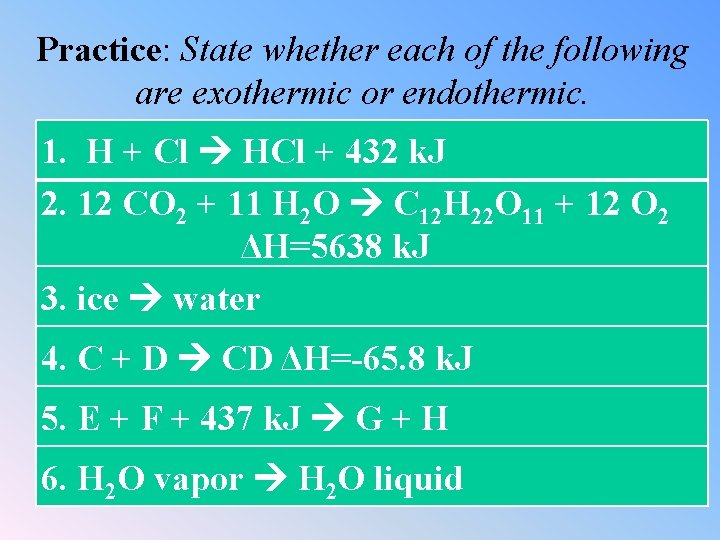
Practice: State whether each of the following are exothermic or endothermic. 1. H + Cl HCl + 432 k. J 2. 12 CO 2 + 11 H 2 O C 12 H 22 O 11 + 12 O 2 ΔH=5638 k. J 3. ice water 4. C + D CD ΔH=-65. 8 k. J 5. E + F + 437 k. J G + H 6. H 2 O vapor H 2 O liquid

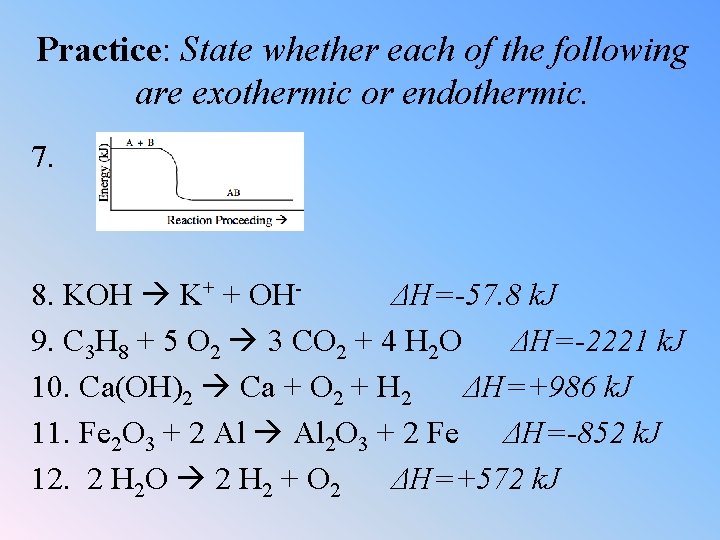
Practice: State whether each of the following are exothermic or endothermic. 7. 8. KOH K+ + OHΔH=-57. 8 k. J 9. C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O ΔH=-2221 k. J 10. Ca(OH)2 Ca + O 2 + H 2 ΔH=+986 k. J 11. Fe 2 O 3 + 2 Al 2 O 3 + 2 Fe ΔH=-852 k. J 12. 2 H 2 O 2 H 2 + O 2 ΔH=+572 k. J

Specific Heat • Know: amount of heat required to raise the temperature of 1 gram by 1°C. • That quantity is defined as the specific heat (c). • Each substance has its own specific heat.

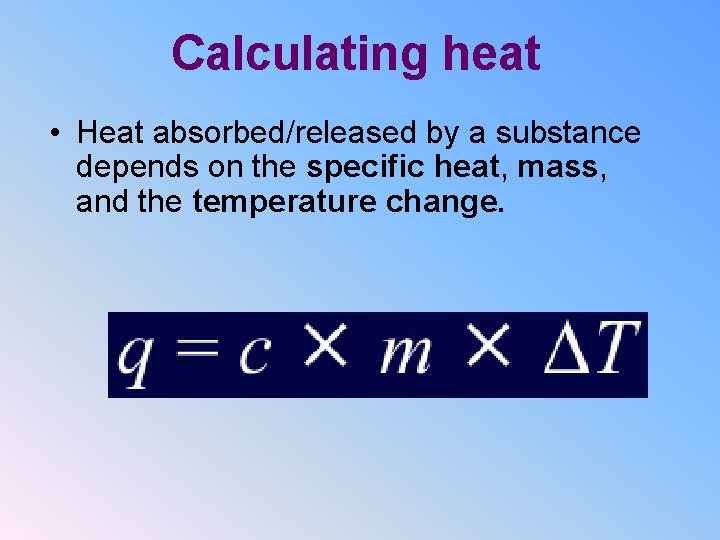
Calculating heat • Heat absorbed/released by a substance depends on the specific heat, mass, and the temperature change.

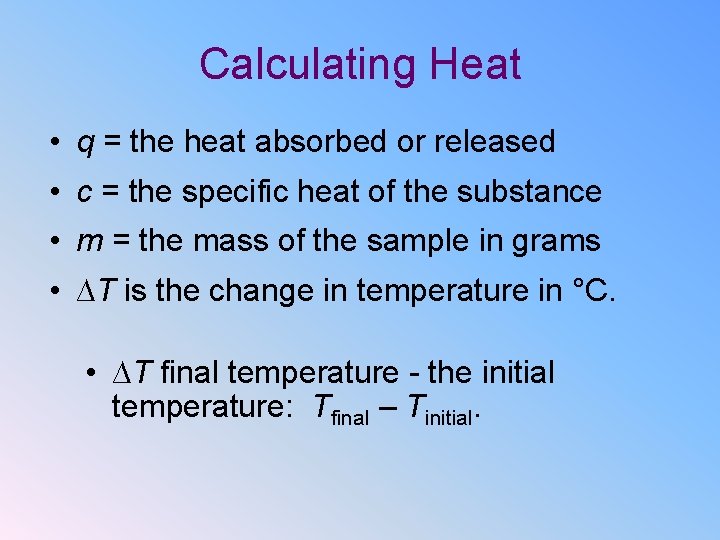
Calculating Heat • q = the heat absorbed or released • c = the specific heat of the substance • m = the mass of the sample in grams • ∆T is the change in temperature in °C. • ∆T final temperature - the initial temperature: Tfinal – Tinitial.

Converting • 256 cal into Jolues • 987 cal into Joules 4 • 5. 9 x 10 J into cal • 3. 7 x 104 J into cal

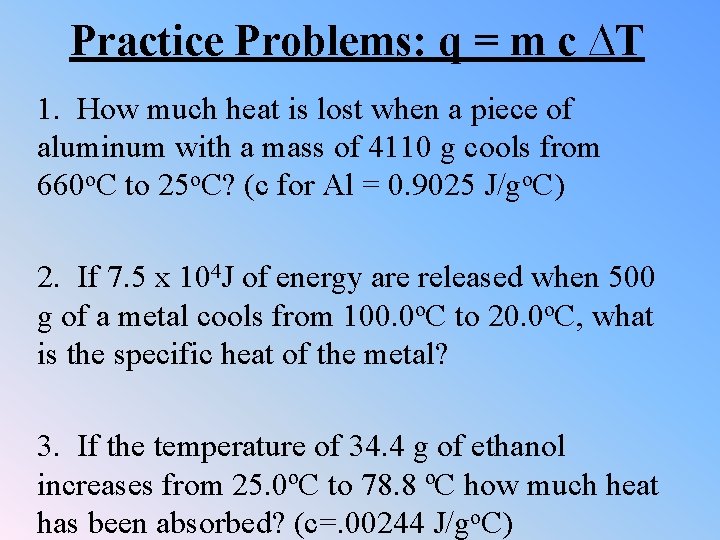
Practice Problems: q = m c ∆T 1. How much heat is lost when a piece of aluminum with a mass of 4110 g cools from 660 o. C to 25 o. C? (c for Al = 0. 9025 J/go. C) 2. If 7. 5 x 104 J of energy are released when 500 g of a metal cools from 100. 0 o. C to 20. 0 o. C, what is the specific heat of the metal? 3. If the temperature of 34. 4 g of ethanol increases from 25. 0ºC to 78. 8 ºC how much heat has been absorbed? (c=. 00244 J/go. C)

Specific Heat Practice Problems 4. A 4. 50 g nugget of gold absorbed 276 J of heat. What was the final temperature of the gold if the initial temperature was 25. 0 ºC and the specific heat is 0. 129 J/g • ºC 5. A 155 g sample of unknown substances was heated from 25. 0 ºC to 40. 0 ºC and it absorbed 5696 J of energy. What is the specific heat of the substance?

Create your own Graphs

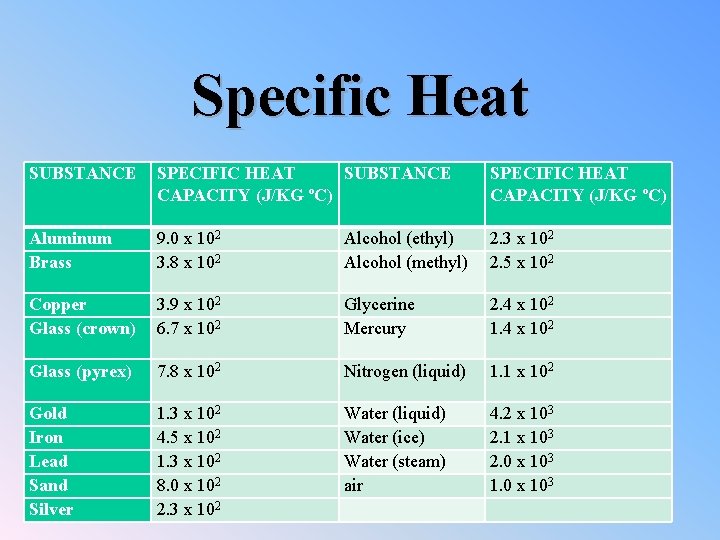
Specific Heat SUBSTANCE SPECIFIC HEAT SUBSTANCE CAPACITY (J/KG ºC) SPECIFIC HEAT CAPACITY (J/KG ºC) Aluminum Brass 9. 0 x 102 3. 8 x 102 Alcohol (ethyl) Alcohol (methyl) 2. 3 x 102 2. 5 x 102 Copper Glass (crown) 3. 9 x 102 6. 7 x 102 Glycerine Mercury 2. 4 x 102 1. 4 x 102 Glass (pyrex) 7. 8 x 102 Nitrogen (liquid) 1. 1 x 102 Gold Iron Lead Sand Silver 1. 3 x 102 4. 5 x 102 1. 3 x 102 8. 0 x 102 2. 3 x 102 Water (liquid) Water (ice) Water (steam) air 4. 2 x 103 2. 1 x 103 2. 0 x 103 1. 0 x 103
 Coffee cup calorimeter vs bomb calorimeter
Coffee cup calorimeter vs bomb calorimeter Coffee cup calorimeter vs bomb calorimeter
Coffee cup calorimeter vs bomb calorimeter Capacity to do work is called
Capacity to do work is called Equivalente meccanico della caloria relazione
Equivalente meccanico della caloria relazione Fluxo de calor
Fluxo de calor 3oz chicken breast
3oz chicken breast Specific heat capacity graph
Specific heat capacity graph Specific latent heat definition
Specific latent heat definition Joule examples
Joule examples Cronies calorie restriction
Cronies calorie restriction Différence entre calorie et kilocalorie
Différence entre calorie et kilocalorie Livestrong calorie counter
Livestrong calorie counter Dragibus soft calories
Dragibus soft calories Calorie orgasmo
Calorie orgasmo Tiramisu light calorie
Tiramisu light calorie Dieta normocalorica esempio
Dieta normocalorica esempio Calorie restriction
Calorie restriction Noci calorie
Noci calorie Tabella laf larn
Tabella laf larn Every calorie counts
Every calorie counts Dieta per menopausa 1200 calorie
Dieta per menopausa 1200 calorie Calcolo fabbisogno calorico giornaliero
Calcolo fabbisogno calorico giornaliero Calorie is defined as
Calorie is defined as Perdite rosa in menopausa
Perdite rosa in menopausa Harris-benedict -basal metabolic rate calorie calc
Harris-benedict -basal metabolic rate calorie calc Type 2 diabetes food chart
Type 2 diabetes food chart Enfamil ar 22 calorie formula recipe
Enfamil ar 22 calorie formula recipe Specific heat equation units
Specific heat equation units Constant volume calorimeter
Constant volume calorimeter Selma abdul
Selma abdul Accelerating rate calorimeter
Accelerating rate calorimeter Now answer these questions
Now answer these questions Constant volume calorimeter
Constant volume calorimeter