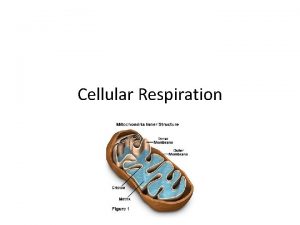
Structure of the adenosine A 2 A receptor





- Slides: 22

Structure of the adenosine A 2 A receptor bound to an engineered G protein B. Carpenter, R. Nehmé, T. Warne, A. G. W. Leslie 1 & C. G. Tate Nature, 2016, 536, 104– 107 姓名 : 施曉慈 學號 : 1013489 2017/05/23 1

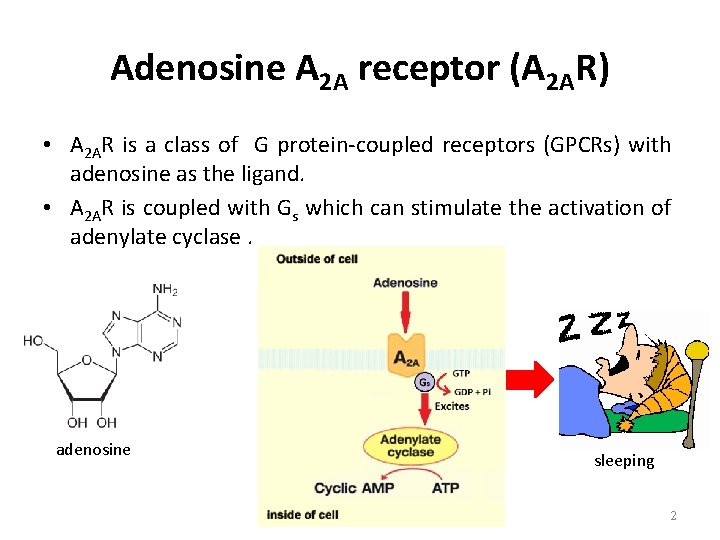
Adenosine A 2 A receptor (A 2 AR) • A 2 AR is a class of G protein-coupled receptors (GPCRs) with adenosine as the ligand. • A 2 AR is coupled with Gs which can stimulate the activation of adenylate cyclase. adenosine sleeping 2

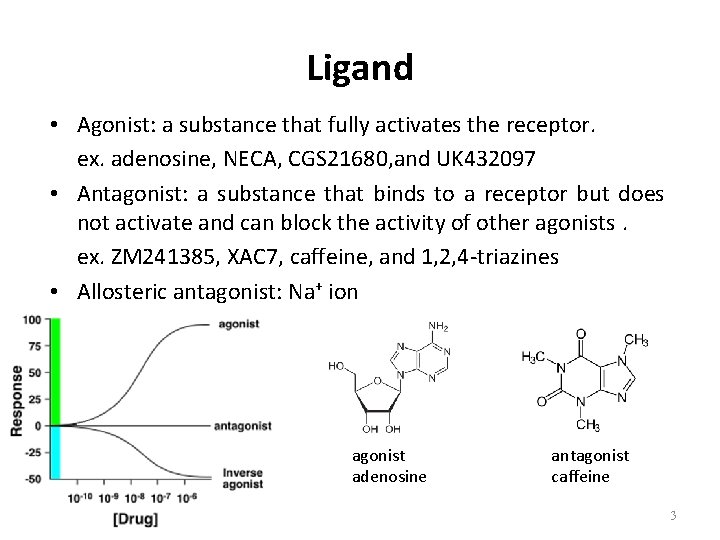
Ligand • Agonist: a substance that fully activates the receptor. ex. adenosine, NECA, CGS 21680, and UK 432097 • Antagonist: a substance that binds to a receptor but does not activate and can block the activity of other agonists. ex. ZM 241385, XAC 7, caffeine, and 1, 2, 4 -triazines • Allosteric antagonist: Na+ ion agonist adenosine antagonist caffeine 3

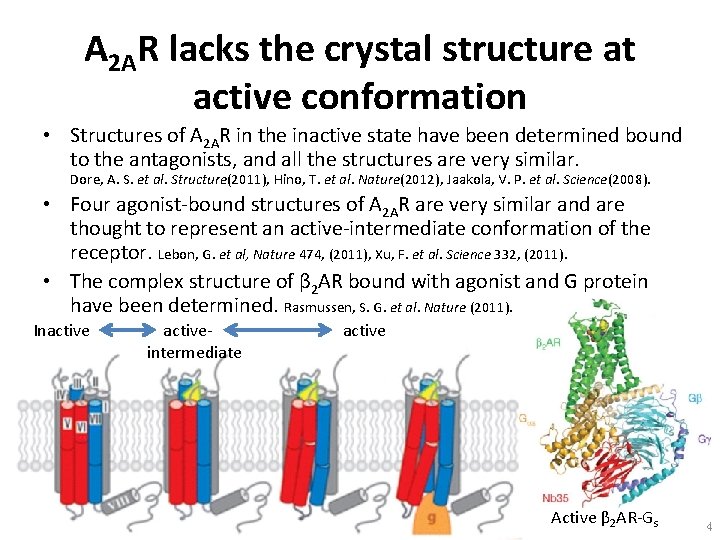
A 2 AR lacks the crystal structure at active conformation • Structures of A 2 AR in the inactive state have been determined bound to the antagonists, and all the structures are very similar. Dore, A. S. et al. Structure(2011), Hino, T. et al. Nature(2012), Jaakola, V. P. et al. Science(2008). • Four agonist-bound structures of A 2 AR are very similar and are thought to represent an active-intermediate conformation of the receptor. Lebon, G. et al, Nature 474, (2011), Xu, F. et al. Science 332, (2011). • The complex structure of β 2 AR bound with agonist and G protein have been determined. Rasmussen, S. G. et al. Nature (2011). Inactive active- active intermediate Active β 2 AR-Gs 4

Motivation • To determine the crystal structure of A 2 AR receptor at active state (agonist-bound). • To understand the conformational influence of the G protein to A 2 AR receptor. 5

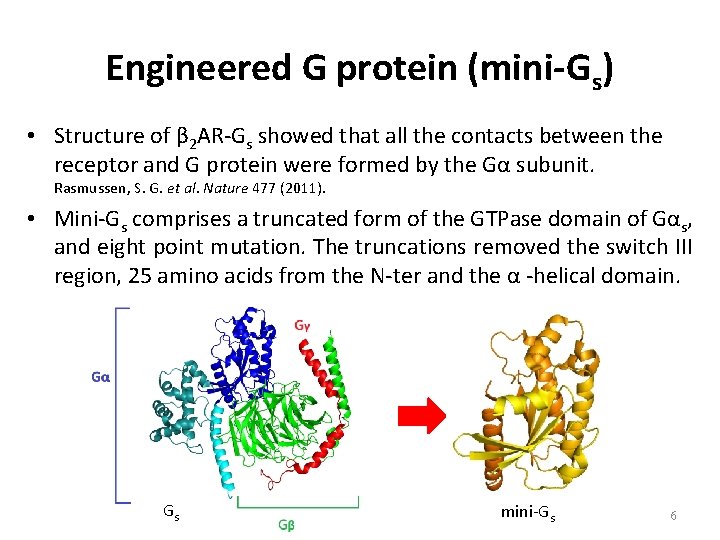
Engineered G protein (mini-Gs) • Structure of β 2 AR-Gs showed that all the contacts between the receptor and G protein were formed by the Gα subunit. Rasmussen, S. G. et al. Nature 477 (2011). • Mini-Gs comprises a truncated form of the GTPase domain of Gαs, and eight point mutation. The truncations removed the switch III region, 25 amino acids from the N-ter and the α -helical domain. Gs mini-Gs 6

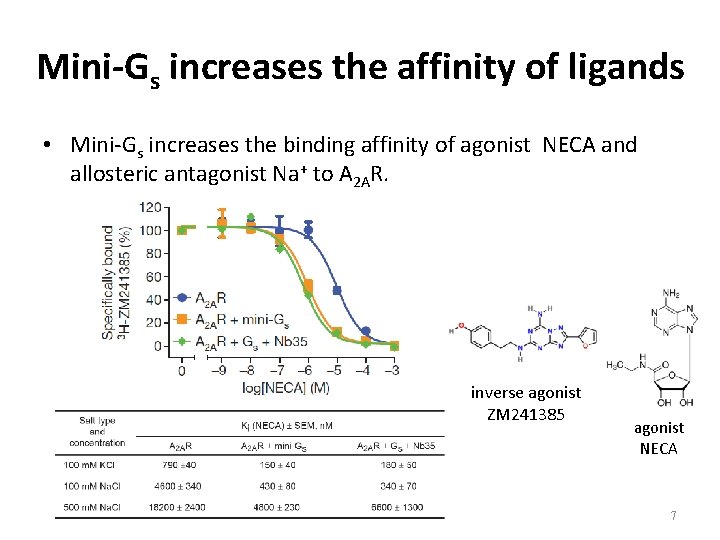
Mini-Gs increases the affinity of ligands • Mini-Gs increases the binding affinity of agonist NECA and allosteric antagonist Na+ to A 2 AR. inverse agonist ZM 241385 agonist NECA 7

Mini-Gs increases thermostability • The complex was considerably more thermostable, particularly in short-chain detergents. 8

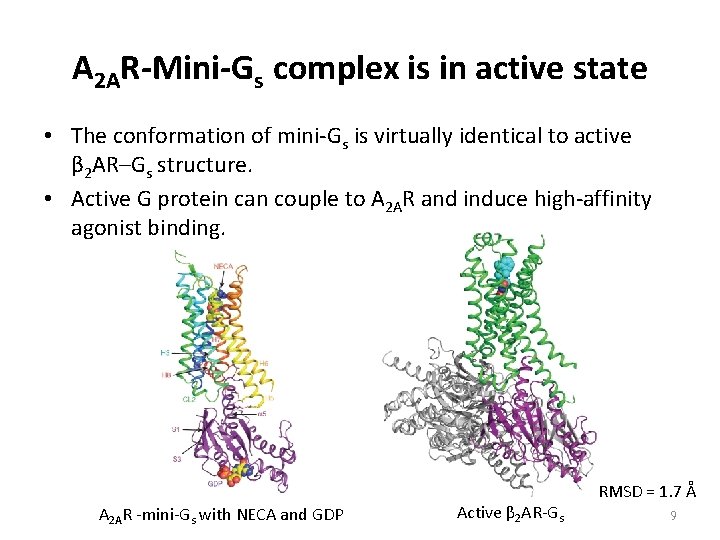
A 2 AR-Mini-Gs complex is in active state • The conformation of mini-Gs is virtually identical to active β 2 AR–Gs structure. • Active G protein can couple to A 2 AR and induce high-affinity agonist binding. A 2 AR -mini-Gs with NECA and GDP Active β 2 AR-Gs RMSD = 1. 7 Å 9

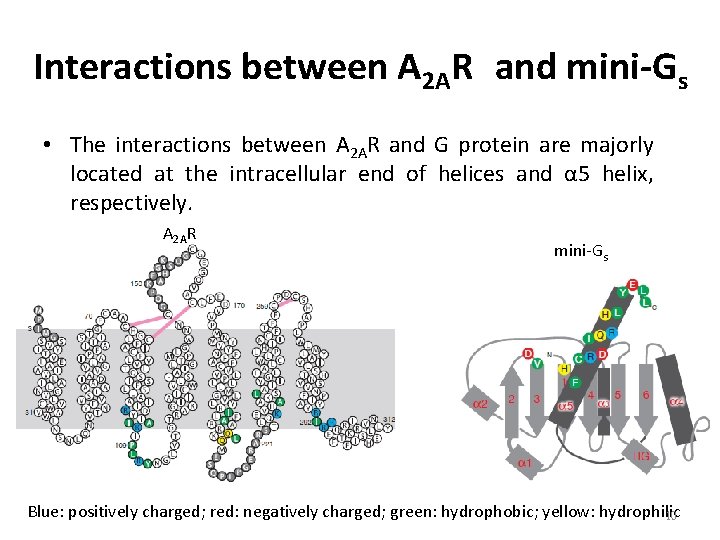
Interactions between A 2 AR and mini-Gs • The interactions between A 2 AR and G protein are majorly located at the intracellular end of helices and α 5 helix, respectively. A 2 AR mini-Gs Blue: positively charged; red: negatively charged; green: hydrophobic; yellow: hydrophilic 10

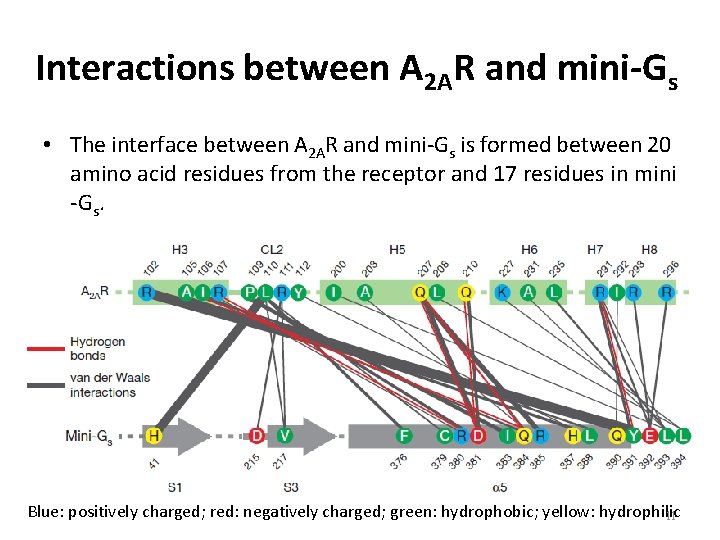
Interactions between A 2 AR and mini-Gs • The interface between A 2 AR and mini-Gs is formed between 20 amino acid residues from the receptor and 17 residues in mini -Gs. Blue: positively charged; red: negatively charged; green: hydrophobic; yellow: hydrophilic 11

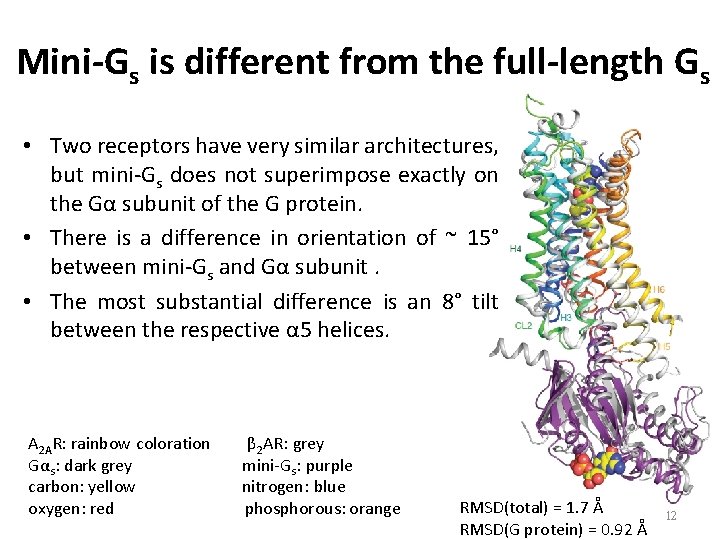
Mini-Gs is different from the full-length Gs • Two receptors have very similar architectures, but mini-Gs does not superimpose exactly on the Gα subunit of the G protein. • There is a difference in orientation of ~ 15° between mini-Gs and Gα subunit. • The most substantial difference is an 8° tilt between the respective α 5 helices. A 2 AR: rainbow coloration β 2 AR: grey Gαs: dark grey mini-Gs: purple carbon: yellow nitrogen: blue oxygen: red phosphorous: orange RMSD(total) = 1. 7 Å RMSD(G protein) = 0. 92 Å 12

Ballesteros–Weinstein numbering and common Gα numbering • The Ballesteros–Weinstein numbering (B-W) and common Gα numbering (CGN) allow relating every amino acid residue position to equivalent positions in homologous proteins. • The most conserved residues are used as anchor point. Ballesteros–Weinstein numbering (GPCR) TM 1 Asn 55 (1. 50) TM 2 Asp 83 (2. 50) TM 3 Arg 135(3. 50) TM 4 Trp 161(4. 50) TM 5 Pro 215(5. 50) TM 6 Pro 267(6. 50) TM 7 Pro 303(7. 50) common Gα numbering (G protein) 13

Hydrogen bonds between A 2 AR receptor and G protein • The 8° tilt between the respective α 5 helices results in a 3. 7 Å displacement of the Cα of Tyr 391 H 5. 23 in mini-Gs. • The interactions between A 2 AR and G protein are highly conserved in β 2 AR-Gs complex. EH 5. 24 14

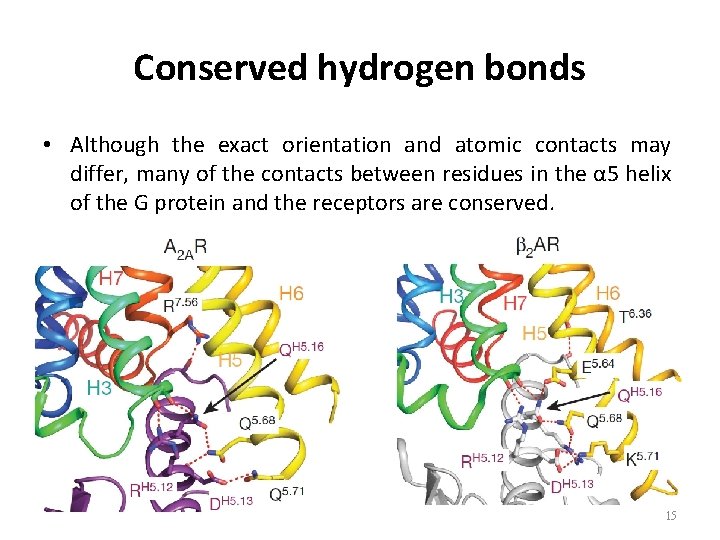
Conserved hydrogen bonds • Although the exact orientation and atomic contacts may differ, many of the contacts between residues in the α 5 helix of the G protein and the receptors are conserved. 15

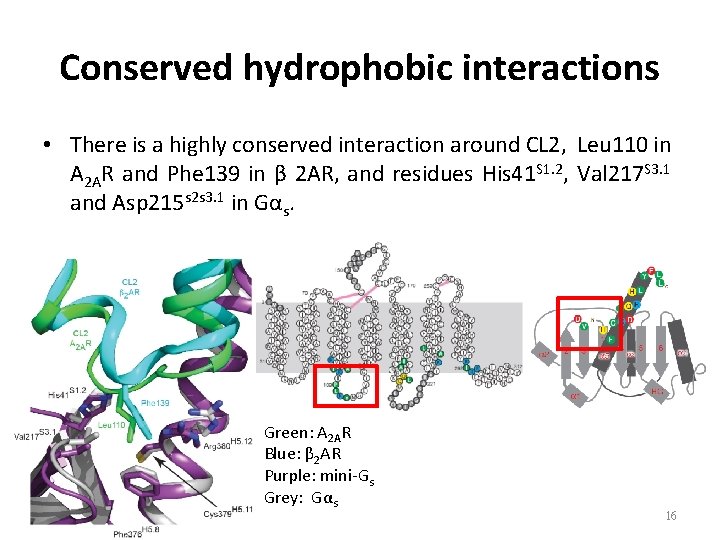
Conserved hydrophobic interactions • There is a highly conserved interaction around CL 2, Leu 110 in A 2 AR and Phe 139 in β 2 AR, and residues His 41 S 1. 2, Val 217 S 3. 1 and Asp 215 s 2 s 3. 1 in Gαs. Green: A 2 AR Blue: β 2 AR Purple: mini-Gs Grey: Gαs 16

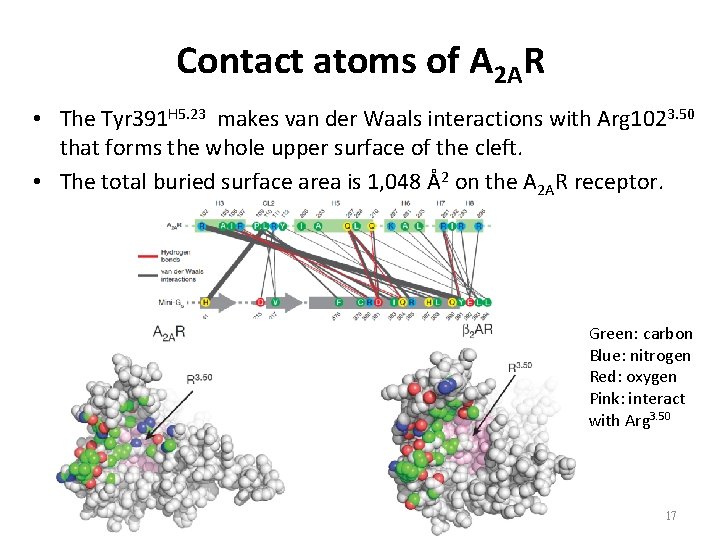
Contact atoms of A 2 AR • The Tyr 391 H 5. 23 makes van der Waals interactions with Arg 1023. 50 that forms the whole upper surface of the cleft. • The total buried surface area is 1, 048 Å2 on the A 2 AR receptor. Green: carbon Blue: nitrogen Red: oxygen Pink: interact with Arg 3. 50 17

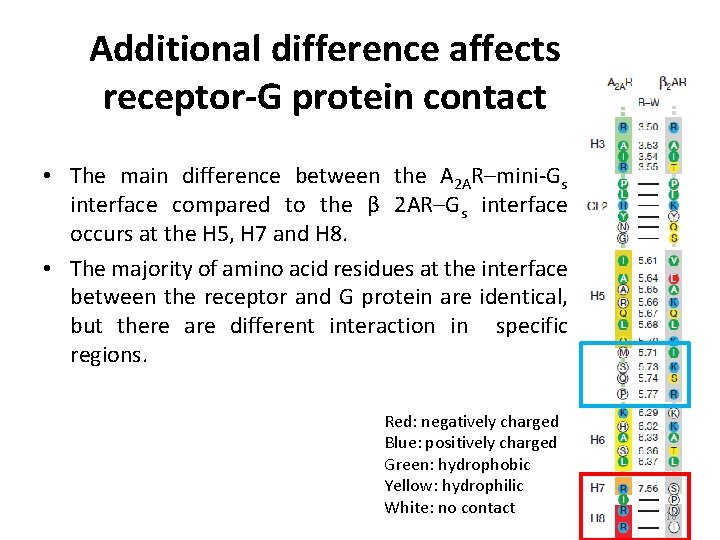
Additional difference affects receptor-G protein contact • The main difference between the A 2 AR–mini-Gs interface compared to the β 2 AR–Gs interface occurs at the H 5, H 7 and H 8. • The majority of amino acid residues at the interface between the receptor and G protein are identical, but there are different interaction in specific regions. Red: negatively charged Blue: positively charged Green: hydrophobic Yellow: hydrophilic White: no contact 18

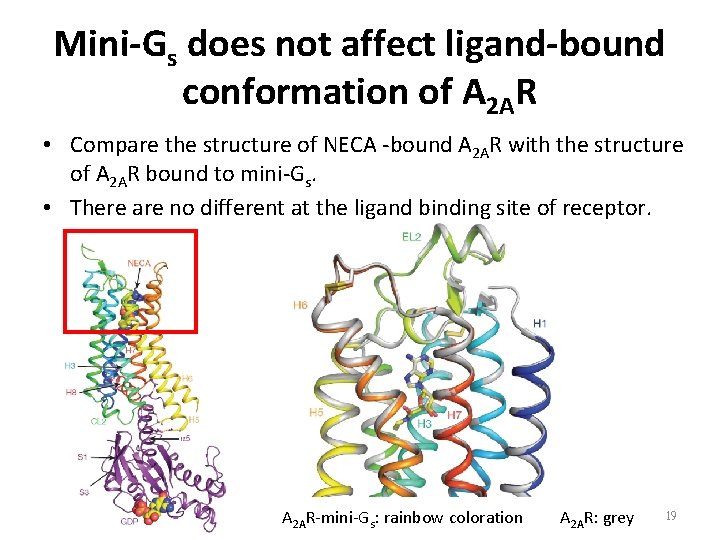
Mini-Gs does not affect ligand-bound conformation of A 2 AR • Compare the structure of NECA -bound A 2 AR with the structure of A 2 AR bound to mini-Gs. • There are no different at the ligand binding site of receptor. A 2 AR-mini-Gs: rainbow coloration A 2 AR: grey 19

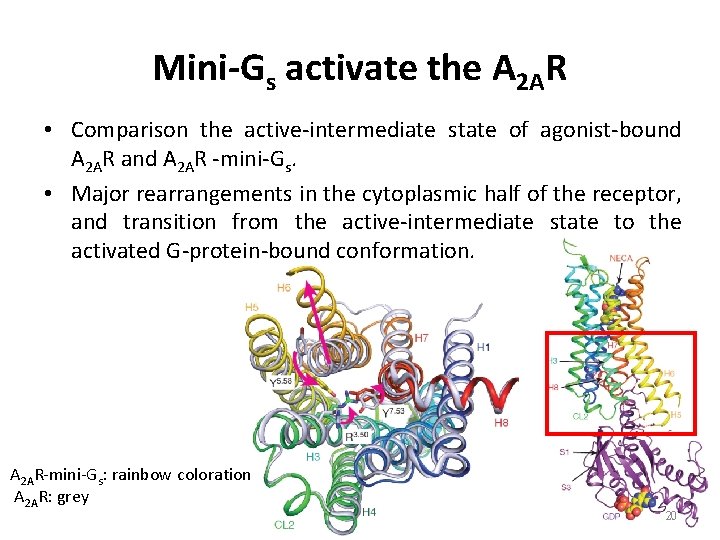
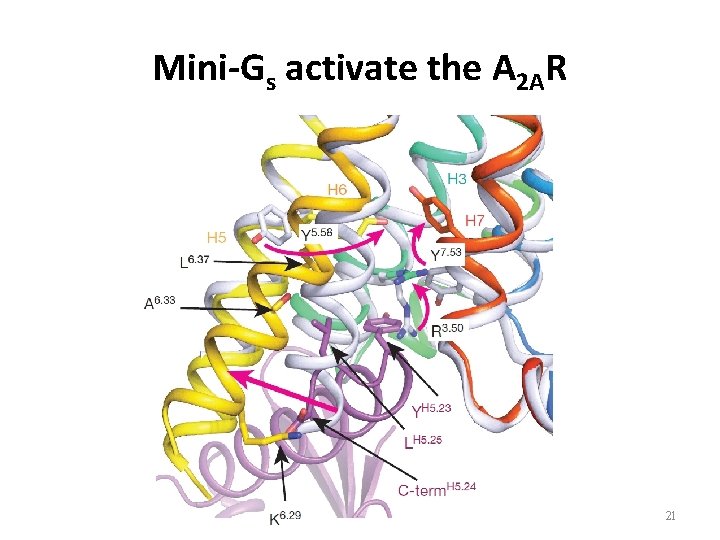
Mini-Gs activate the A 2 AR • Comparison the active-intermediate state of agonist-bound A 2 AR and A 2 AR -mini-Gs. • Major rearrangements in the cytoplasmic half of the receptor, and transition from the active-intermediate state to the activated G-protein-bound conformation. A 2 AR-mini-Gs: rainbow coloration A 2 AR: grey 20

Mini-Gs activate the A 2 AR 21

Conclusion • The transition of the receptor from agonist-bound activeintermediate state to active state has the following characteristics: – 14 Å shift of the cytoplasmic end H 6 away from the receptor core – slight changes in the cytoplasmic ends of H 5 and H 7 – rotamer changes of the amino acid side chains Arg 3. 50, Tyr 5. 58 and Tyr 7. 53 22
 Adenosine vs amiodarone
Adenosine vs amiodarone Adenosine triphosphate (atp)
Adenosine triphosphate (atp) Adenosine ribonucleotide
Adenosine ribonucleotide Adenosine
Adenosine Factorig
Factorig Receptor tyrosine kinase structure
Receptor tyrosine kinase structure Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Chúa sống lại
Chúa sống lại Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống