Stoichiometry The Mole Stoichiometry The study of the







- Slides: 21

Stoichiometry: The Mole

Stoichiometry The study of the quantitative aspects of chemical reactions.

“The Mole” • A counting unit • 1 mole = 602 billion trillion: 602, 000, 000, 000 • 6. 02 X 1023 (in scientific notation) • 1 dozen eggs = 12 • 1 “mole” of eggs = 6. 02 x 1023 eggs • 1 dozen cars = 12 cars • 1 “mole” of cars = 6. 02 x 1023 cars Note that the NUMBER is always the same, but the MASS is very different! Mole is abbreviated “mol”

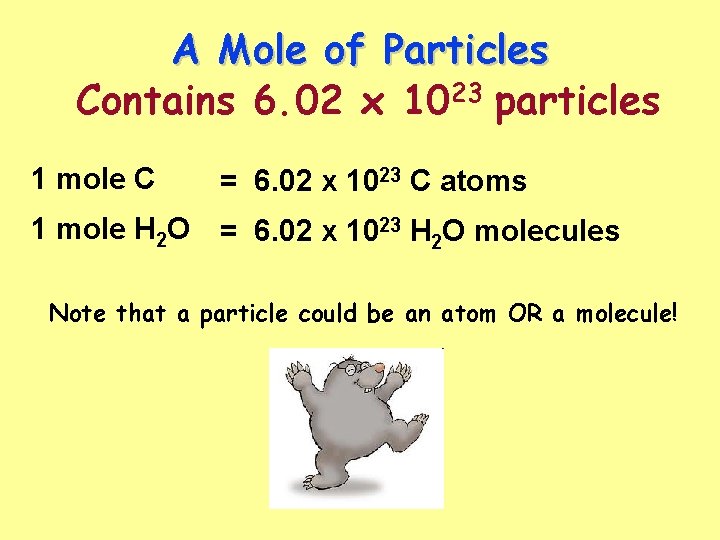
A Mole of Particles Contains 6. 02 x 1023 particles 1 mole C = 6. 02 x 1023 C atoms 1 mole H 2 O = 6. 02 x 1023 H 2 O molecules Note that a particle could be an atom OR a molecule!

Avogadro’s Number as a Conversion Factor 6. 02 x 1023 particles 1 mole or 1 mole 6. 02 x 1023 particles

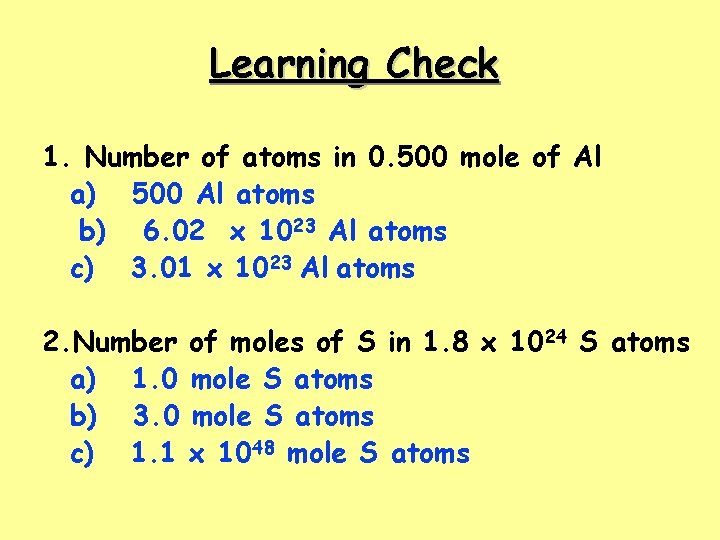
Learning Check 1. Number of atoms in 0. 500 mole of Al a) 500 Al atoms b) 6. 02 x 1023 Al atoms c) 3. 01 x 1023 Al atoms 2. Number a) 1. 0 b) 3. 0 c) 1. 1 of moles of S in 1. 8 x 1024 S atoms mole S atoms x 1048 mole S atoms

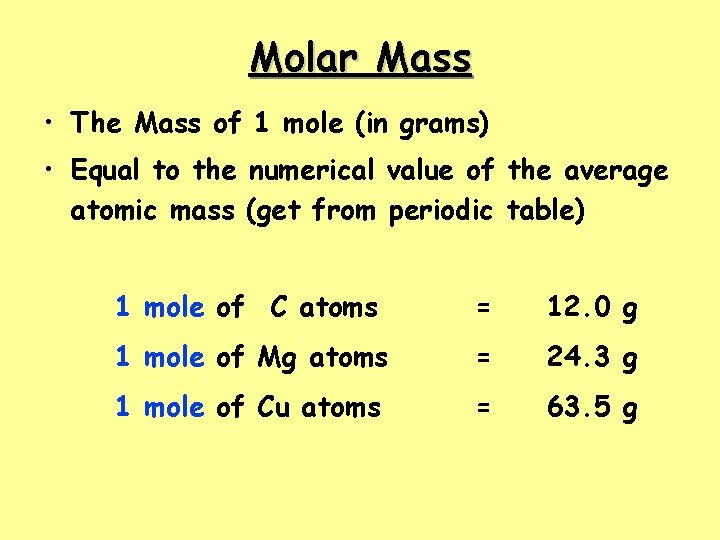
Molar Mass • The Mass of 1 mole (in grams) • Equal to the numerical value of the average atomic mass (get from periodic table) 1 mole of C atoms = 12. 0 g 1 mole of Mg atoms = 24. 3 g 1 mole of Cu atoms = 63. 5 g

Learning Check! Find the molar mass: (usually we round to the tenths place) A. 1 mole of Br atoms = B. 1 mole of Sn atoms= 79. 9 g/mole 118. 7 g/mole

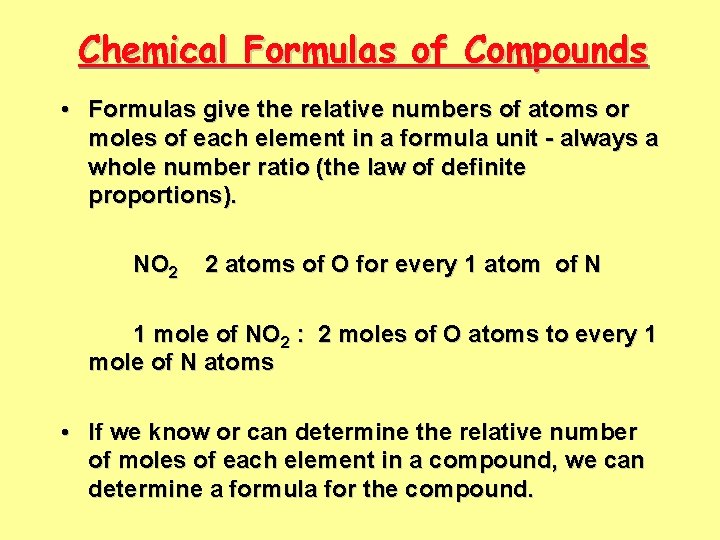
Chemical Formulas of Compounds • Formulas give the relative numbers of atoms or moles of each element in a formula unit - always a whole number ratio (the law of definite proportions). NO 2 2 atoms of O for every 1 atom of N 1 mole of NO 2 : 2 moles of O atoms to every 1 mole of N atoms • If we know or can determine the relative number of moles of each element in a compound, we can determine a formula for the compound.

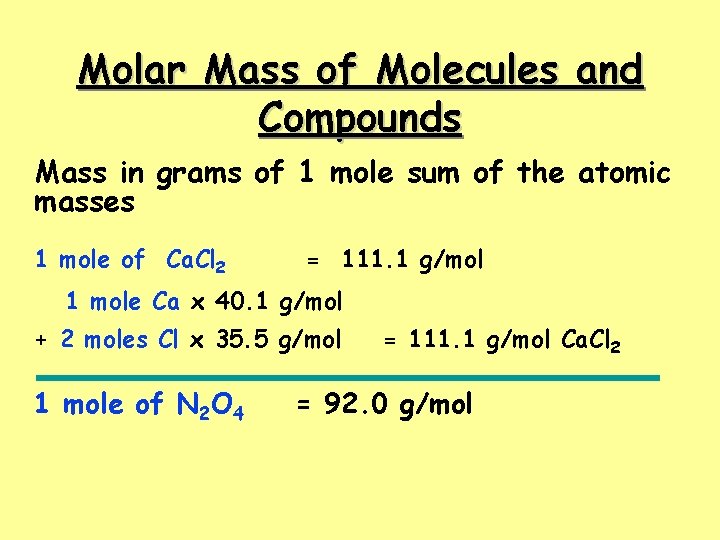
Molar Mass of Molecules and Compounds Mass in grams of 1 mole sum of the atomic masses 1 mole of Ca. Cl 2 = 111. 1 g/mol 1 mole Ca x 40. 1 g/mol + 2 moles Cl x 35. 5 g/mol 1 mole of N 2 O 4 = 111. 1 g/mol Ca. Cl 2 = 92. 0 g/mol

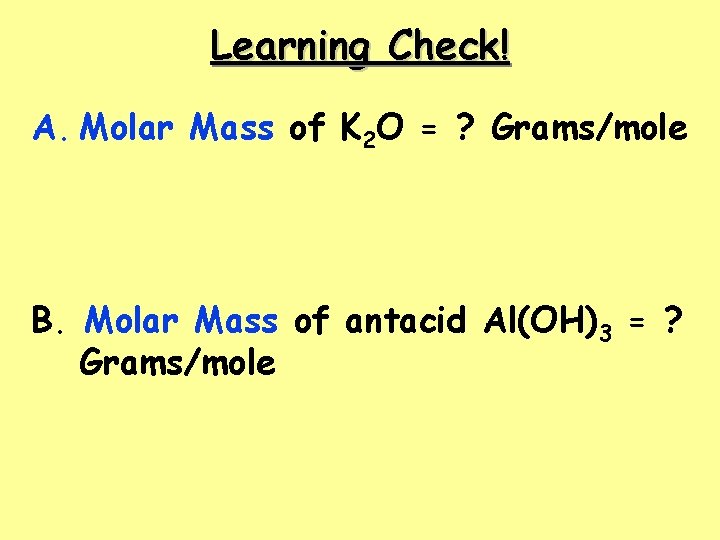
Learning Check! A. Molar Mass of K 2 O = ? Grams/mole B. Molar Mass of antacid Al(OH)3 = ? Grams/mole

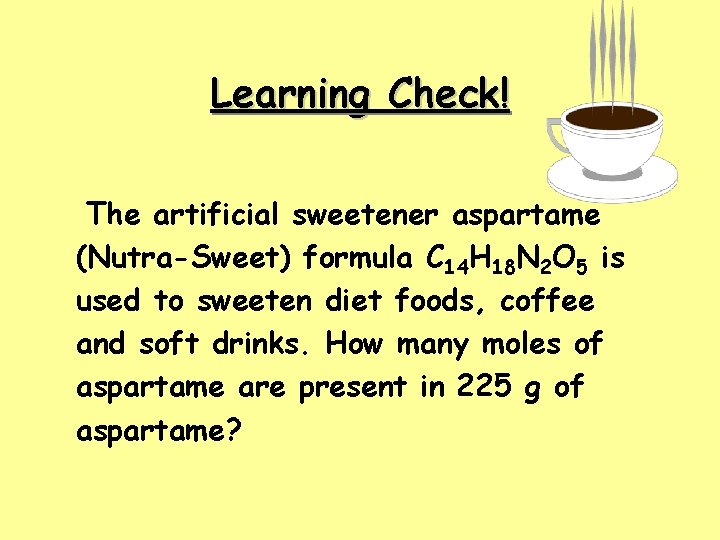
Learning Check! The artificial sweetener aspartame (Nutra-Sweet) formula C 14 H 18 N 2 O 5 is used to sweeten diet foods, coffee and soft drinks. How many moles of aspartame are present in 225 g of aspartame?

Calculations molar mass Grams Avogadro’s number Moles particles You can convert Grams to Particles, but you always must go through moles first.

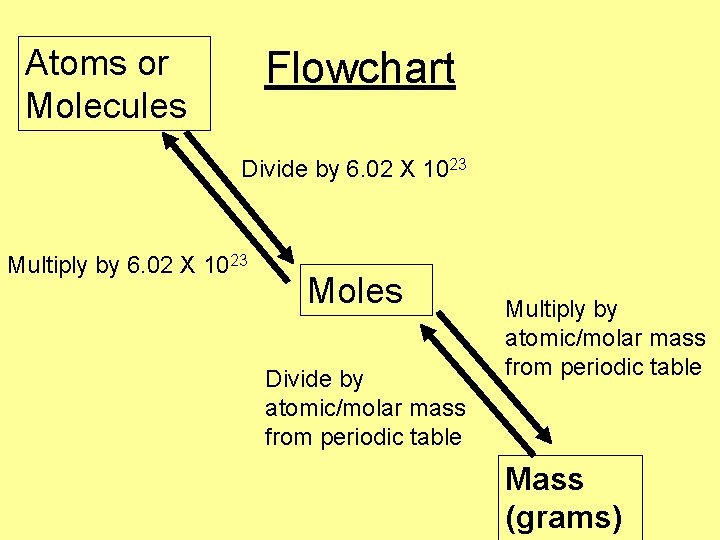
Atoms or Molecules Flowchart Divide by 6. 02 X 1023 Multiply by 6. 02 X 1023 Moles Divide by atomic/molar mass from periodic table Multiply by atomic/molar mass from periodic table Mass (grams)

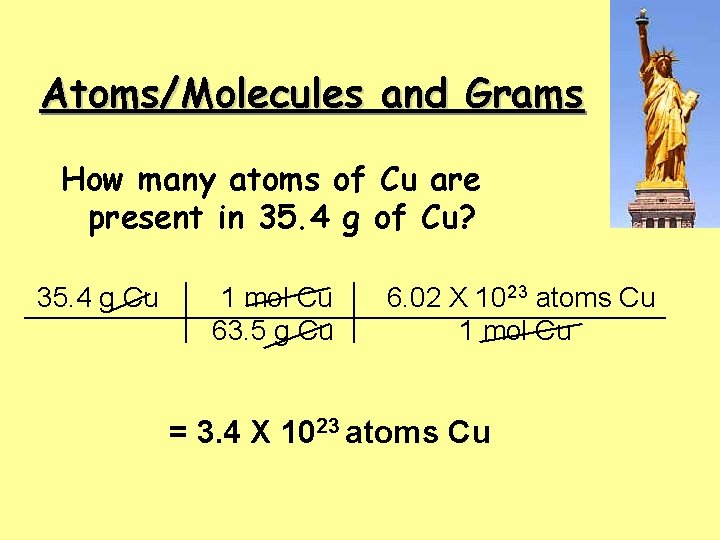
Atoms/Molecules and Grams How many atoms of Cu are present in 35. 4 g of Cu? 35. 4 g Cu 1 mol Cu 63. 5 g Cu 6. 02 X 1023 atoms Cu 1 mol Cu = 3. 4 X 1023 atoms Cu

Learning Check! How many atoms of K are present in 78. 4 g of K?

Learning Check! What is the mass (in grams) of 1. 20 x 1024 molecules of glucose (C 6 H 12 O 6)?

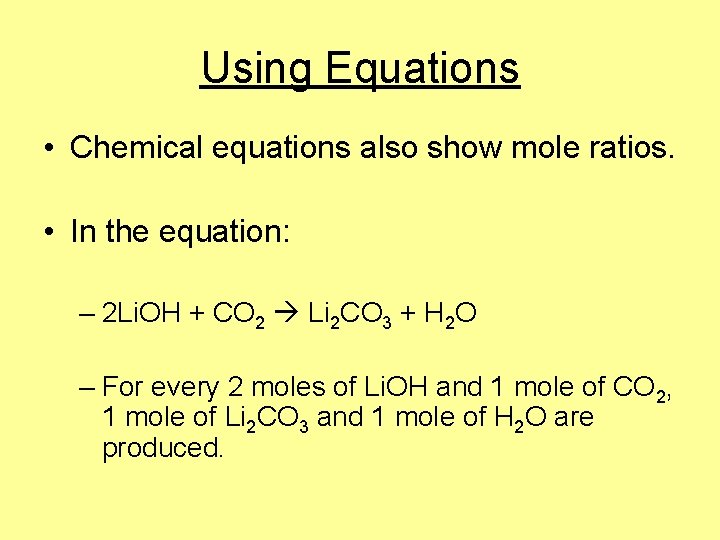
Using Equations • Chemical equations also show mole ratios. • In the equation: – 2 Li. OH + CO 2 Li 2 CO 3 + H 2 O – For every 2 moles of Li. OH and 1 mole of CO 2, 1 mole of Li 2 CO 3 and 1 mole of H 2 O are produced.

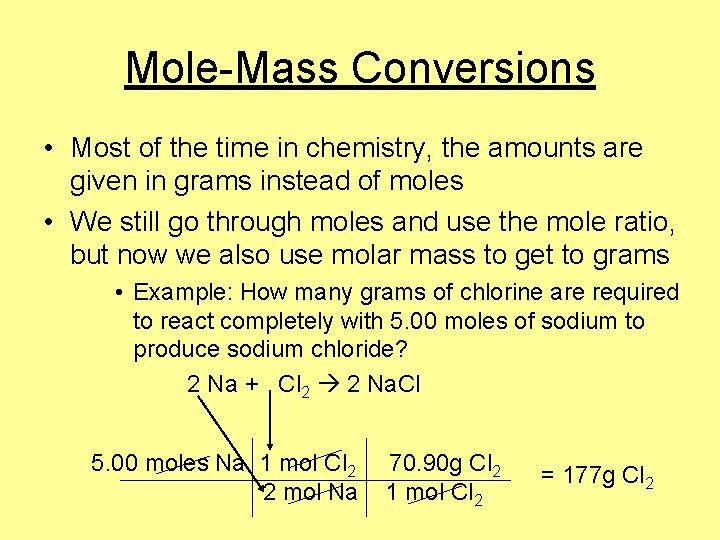
Mole-Mass Conversions • Most of the time in chemistry, the amounts are given in grams instead of moles • We still go through moles and use the mole ratio, but now we also use molar mass to get to grams • Example: How many grams of chlorine are required to react completely with 5. 00 moles of sodium to produce sodium chloride? 2 Na + Cl 2 2 Na. Cl 5. 00 moles Na 1 mol Cl 2 2 mol Na 70. 90 g Cl 2 1 mol Cl 2 = 177 g Cl 2

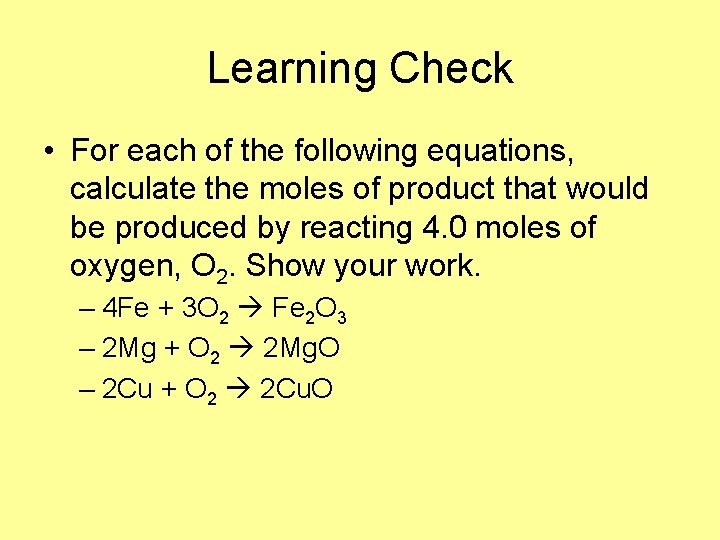
Learning Check • For each of the following equations, calculate the moles of product that would be produced by reacting 4. 0 moles of oxygen, O 2. Show your work. – 4 Fe + 3 O 2 Fe 2 O 3 – 2 Mg + O 2 2 Mg. O – 2 Cu + O 2 2 Cu. O

Learning Check • Given the following reaction: – N 2 + O 2 2 NO a) How many moles of NO can form when 40. 0 g of N 2 react with excess oxygen? b) How many grams of NO can form, using the amounts in part A?
 Stoichiometry: mole-mole problems
Stoichiometry: mole-mole problems Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Phosphorus + oxygen
Phosphorus + oxygen Stoichiometry mole-mole
Stoichiometry mole-mole Mole-mole factor
Mole-mole factor Mol.si
Mol.si Mole mass and mole volume relationships
Mole mass and mole volume relationships Mole tunnel stoichiometry
Mole tunnel stoichiometry Stoichiometry island diagram
Stoichiometry island diagram Stoichiometry island
Stoichiometry island Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Chapter 10 chemistry study guide
Chapter 10 chemistry study guide Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Stoichiometry
Stoichiometry Chapter 11 assessment chemistry
Chapter 11 assessment chemistry Stoichiometry is the study of
Stoichiometry is the study of Marty lobdell
Marty lobdell Ecological study vs cohort study
Ecological study vs cohort study Phytogeographical regions of india map
Phytogeographical regions of india map Retrospective cohort study
Retrospective cohort study Objectives of work study
Objectives of work study Work study technique
Work study technique