States of Matter Introduction to Chemistry Chemistry The











- Slides: 23

States of Matter

Introduction to Chemistry �Chemistry –The study of the properties of matter and how matter changes �Matter -Anything that has mass and takes up space (that space is volume)

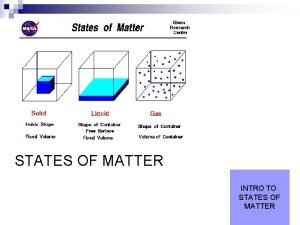
Most Common Phases of Matter �Solid �Liquid �Gas How can matter be described? State Color Texture Odor Measurements (volume, mass, density)

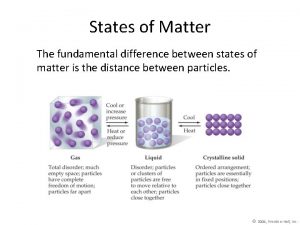
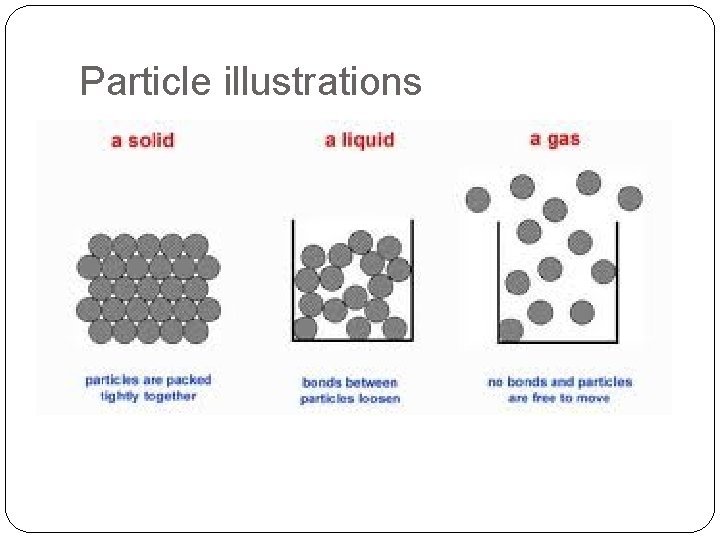
Solids �Matter that has definite shape and definite volume. �Arrangement of Particles: Very close together �How do these particles move? Vibrate in place �Illustration of particle arrangement.

Liquid �Matter that has no definite shape but has a definite volume. Further apart of than particles in a solid (greater �Arrangement Particles: potential energy) Moredo freely. shape of �How these. Particles particlestake move? container. �Illustration of particle arrangement.

Gas �Matter that has no definite shape or no definite volume. Large distance �Arrangement of between Particles: particles. Greatest amount of potential energy. Quickly and freely. �How do these particles move? �Illustration of particle arrangement.

Particle illustrations

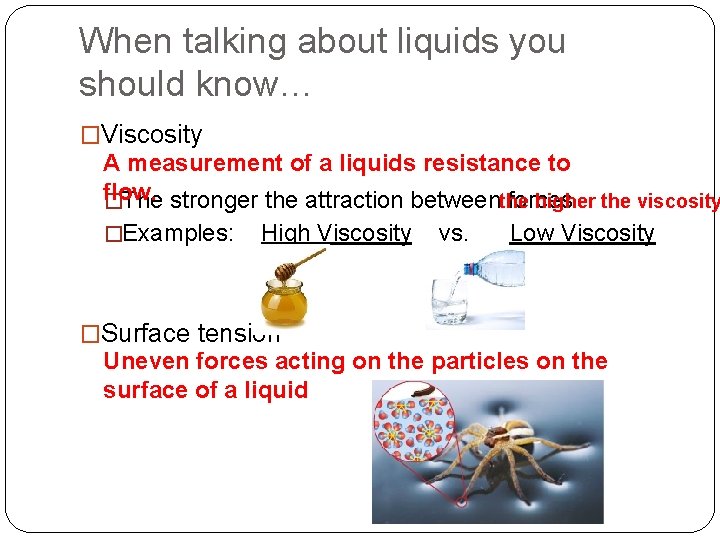
When talking about liquids you should know… �Viscosity A measurement of a liquids resistance to flow. higher the viscosity �The stronger the attraction betweenthe forces �Examples: High Viscosity �Surface tension vs. Low Viscosity Uneven forces acting on the particles on the surface of a liquid

When talking about gas you should know… �Vapor The gas state of a substance that is normally a solid or a liquid at room temperature. �Evaporation Vaporization that occurs only at the surface of a liquid.

Changing an objects state �You can change an objects state of matter by energy adding orthermal removing �When you add thermal energy: The particles move faster (increased kinetic energy) or move farther apart (increased potential energy) or both. �When you remove thermal energy the opposite occurs: Particles slow down (kinetic energy decreases) or move closer together (decrease potential energy) or both.

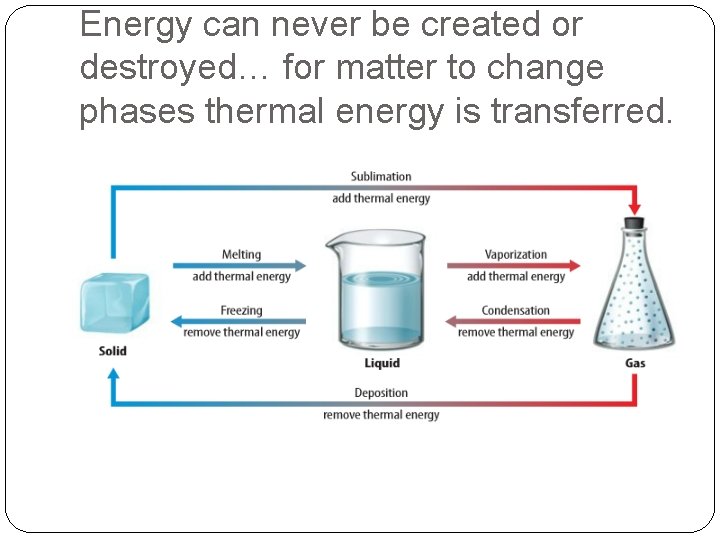
Solid to Liquid or Liquid to Solid �Melting To change matter from a solid to a liquid. Thermal energy must be added. �Freezing To change matter from a liquid to a solid. Thermal energy must be removed.

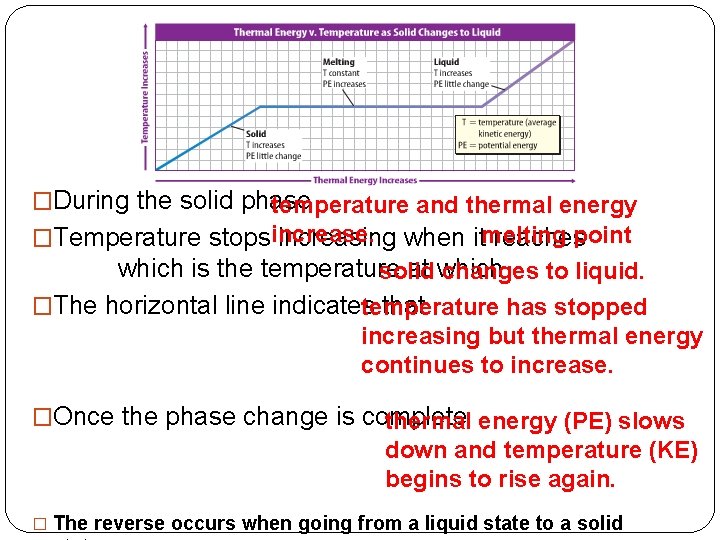
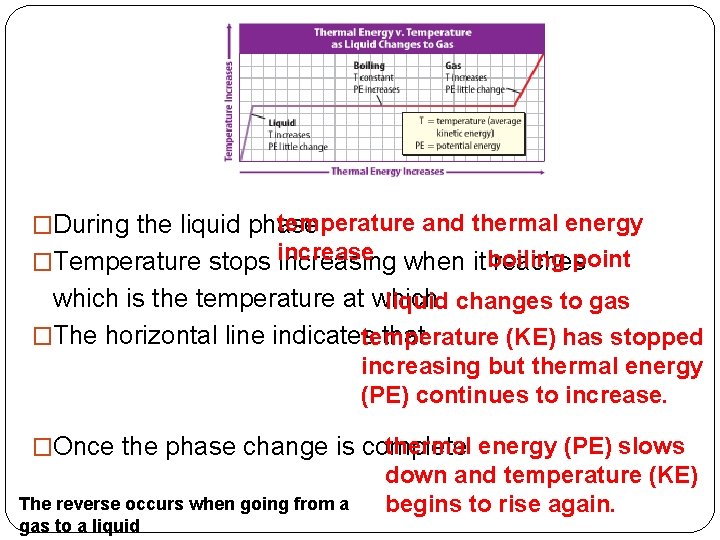
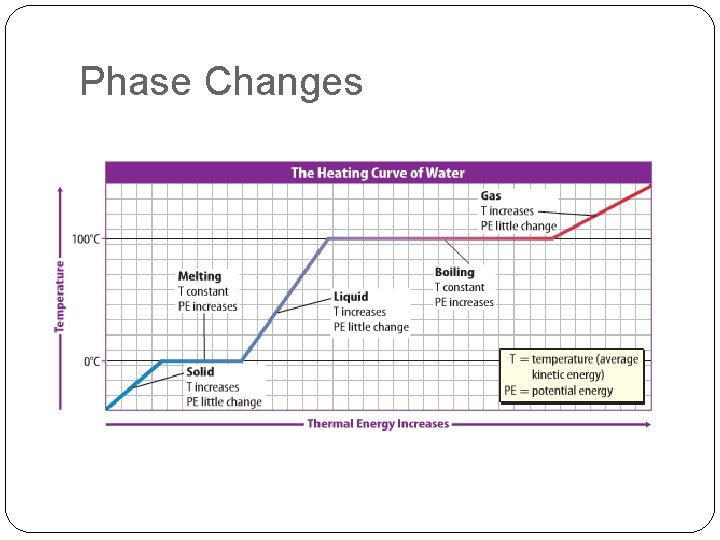
�During the solid phase temperature and thermal energy �Temperature stopsincrease. increasing when itmelting reachespoint which is the temperature at which solid changes to liquid. �The horizontal line indicates that temperature has stopped increasing but thermal energy continues to increase. �Once the phase change is complete thermal energy (PE) slows down and temperature (KE) begins to rise again. � The reverse occurs when going from a liquid state to a solid

Liquid to Gas or Gas to Liquid �Boiling Vaporization that occurs throughout a liquid. Changes liquid to a gas. �Condensation The change of state from a gas to a liquid. When vapor cools back to a liquid.

What is the main difference between evaporation and boiling? During evaporation a substance vaporizes only at the surface. During boiling a substance vaporizes at the surface but also within the liquid.

temperature and thermal energy �During the liquid phase point �Temperature stops increase increasing when it boiling reaches which is the temperature at which liquid changes to gas �The horizontal line indicates that temperature (KE) has stopped increasing but thermal energy (PE) continues to increase. thermal energy (PE) slows �Once the phase change is complete The reverse occurs when going from a gas to a liquid down and temperature (KE) begins to rise again.

Is it possible for a solid to become a gas without becoming a liquid first? Sublimation �YES! This is called �Sublimation The change of state from a solid to a gas without going through the liquid state �Deposition The change of state of a gas to a solid without going through the liquid state.

Phase Changes

Energy can never be created or destroyed… for matter to change phases thermal energy is transferred.

Lesson 3: Behavior of Gases

Pressure and Volume �Pressure The amount of force applied per unit of area. �Volume The amount of space an object occupies.

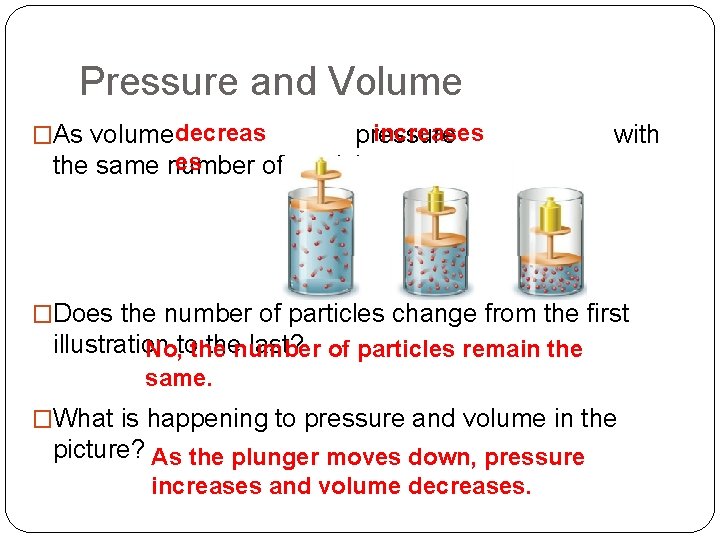
Pressure and Volume �As volume decreas increases pressure es the same number of particles. with �Does the number of particles change from the first illustration thenumber last? of particles remain the No, tothe same. �What is happening to pressure and volume in the picture? As the plunger moves down, pressure increases and volume decreases.

Boyle’s Law �Pressure of a gas increases if the volume decreases and pressure of a gas decreases if the volume increases when the temperature is constant. British scientist who was the first to describe the �Robert Boyle properties of gases. �Have your ears ever popped in an airplane or in the car? (at Use Boyle’s Law to explain how this On the ground sea level) air pressure inside your ear and the pressure of the air surrounding your ear are equal. happens. When you increase altitude air pressure surrounding your ear decreases while the pressure in your ear stays the same causing increase in volume. This causes the pain

Charles’s Law �The volume of a gas increases with increasing temperature, if the pressure is constant. �Jacque Charles French scientist who described the relationship between temperature and volume of gas. �What happens to a full balloon if you take it outside on a cold winter day? When the balloon is in the cold air, the temperature of the gas inside the balloon decreases, causing the particles to slow down and come close together. Fewer particles hit the inside of the balloon causing it to look deflated.
 Phases of matter foldable
Phases of matter foldable Four states of matter
Four states of matter Four states of matter
Four states of matter States of matter
States of matter Which state of matter has the most thermal energy
Which state of matter has the most thermal energy Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Metal non metal liquid venn diagram
Metal non metal liquid venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that 11 free states
11 free states Southern states vs northern states
Southern states vs northern states Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter: basics
States of matter: basics States of matter foldable
States of matter foldable Thermal energy in states of matter
Thermal energy in states of matter Use of heat
Use of heat The fundamental difference between states of matter is the
The fundamental difference between states of matter is the Stayes of matter
Stayes of matter Solid to plasma
Solid to plasma Meysam golmohammadi
Meysam golmohammadi Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter