States of Matter I What is Matter Matter











- Slides: 18

States of Matter

I. What is Matter? • Matter is anything that has mass and occupies space. -Science Textbook

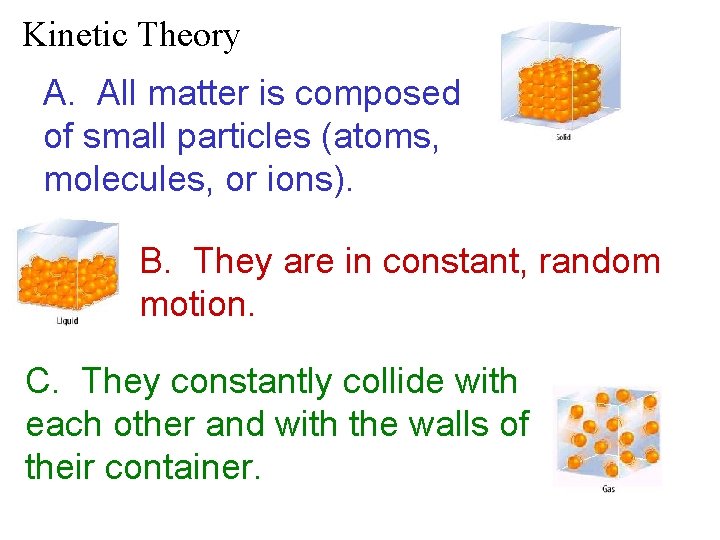
Kinetic Theory A. All matter is composed of small particles (atoms, molecules, or ions). B. They are in constant, random motion. C. They constantly collide with each other and with the walls of their container.

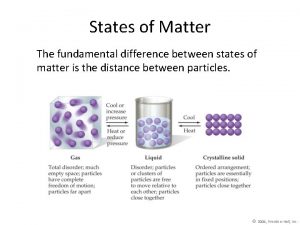
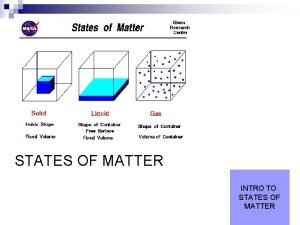
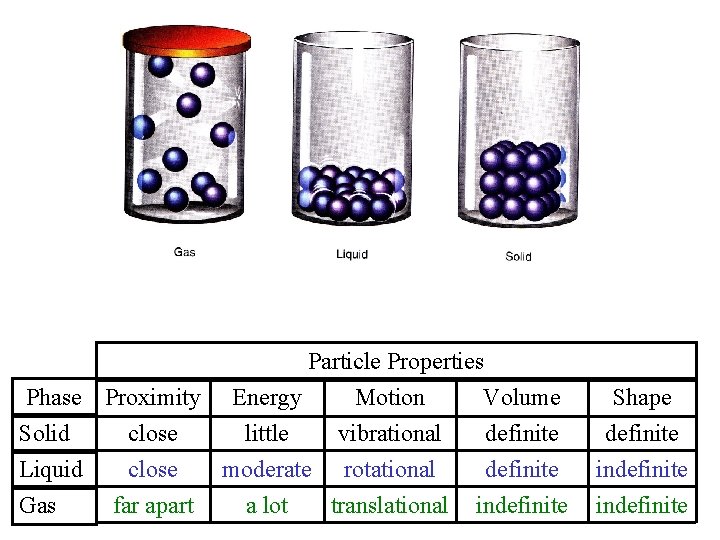
II. Particle Motion • Particles in a: – gas are well separated with no regular arrangement. – liquid are close together with no regular arrangement. – solid are tightly packed, usually in a regular pattern. • Particles in a: – gas vibrate and move freely at high speeds. – liquid vibrate, move about, and slide past each other. – solid vibrate (jiggle) but generally do not move from place to place.

B. Pictures Click here to see the atoms in motion.

III. States of Water A. Water is a molecule made of 1 oxygen atom and 2 hydrogen atoms H 20.

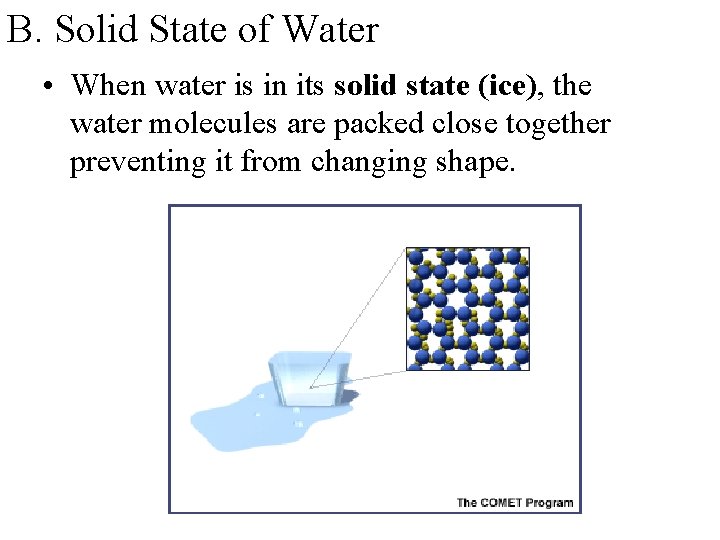
B. Solid State of Water • When water is in its solid state (ice), the water molecules are packed close together preventing it from changing shape.

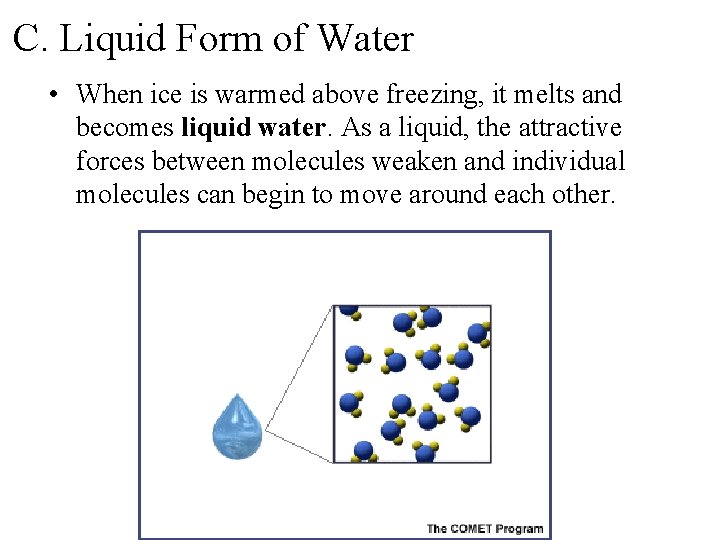
C. Liquid Form of Water • When ice is warmed above freezing, it melts and becomes liquid water. As a liquid, the attractive forces between molecules weaken and individual molecules can begin to move around each other.

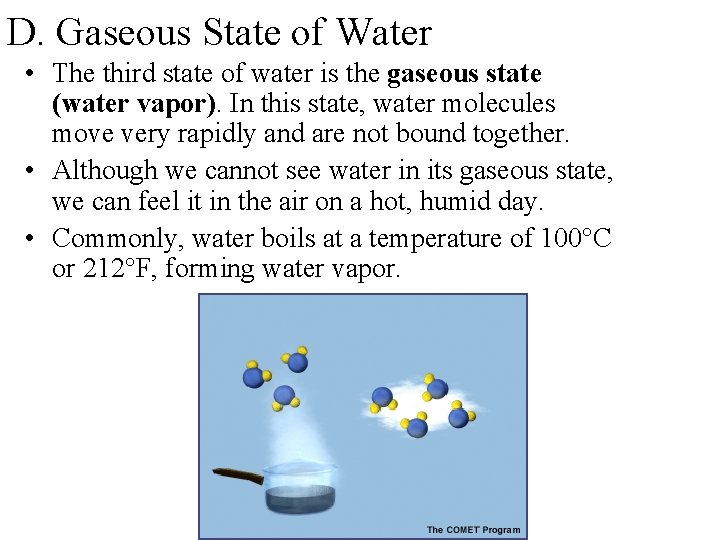
D. Gaseous State of Water • The third state of water is the gaseous state (water vapor). In this state, water molecules move very rapidly and are not bound together. • Although we cannot see water in its gaseous state, we can feel it in the air on a hot, humid day. • Commonly, water boils at a temperature of 100°C or 212°F, forming water vapor.

Particle Properties Phase Proximity Energy Motion Volume Solid close little vibrational definite Liquid close moderate rotational definite Gas far apart a lot translational indefinite Shape definite indefinite

IV. Changing States of Matter A. Melting - solid to liquid • Particles get more kinetic energy and begin rotating around each other.

B. Freezing - liquid to solid • Particles lose kinetic energy and slow down. • Attractive forces between particles become stronger than the particles’ motion, so the particles begin merely vibrating in place.

C. Vaporization - liquid to gas Types: a) Boiling - rapid; gas bubbles are produced throughout. b) Evaporation - slow; occurs at the surface.

1. Evaporation is a cooling process. a. Particles in a liquid gain kinetic energy. b. They leave as gas particles (taking the energy away with them). c. This leaves less energy in the liquid, therefore cooling down what is left.

D. Condensation - gas to liquid • Particles lose kinetic energy, slow down, and come closer together.

E. Sublimation - solid to gas or gas to solid a. Dry ice - carbon dioxide b. Iodine c. Frost Picture found at : www. ice-agency. com

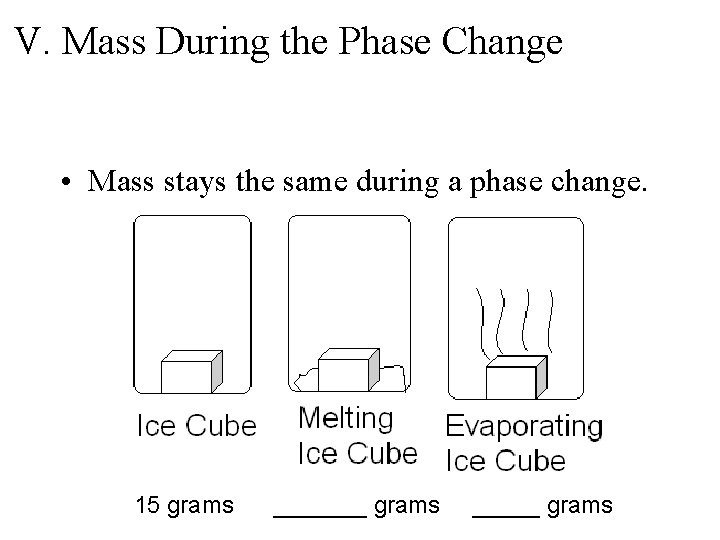
V. Mass During the Phase Change • Mass stays the same during a phase change. 15 grams _______ grams

Resources • http: //www. ucar. edu/learn/1_1_2_3 t. htm • http: //www. chem. purdue. edu/gchelp/atoms/ states. html
 11 free states
11 free states Southern states
Southern states Big states vs small states guard against tyranny
Big states vs small states guard against tyranny 5 states of matter
5 states of matter States of matter
States of matter Uses of heat
Uses of heat 5 states of matter
5 states of matter Four states of matter
Four states of matter 5 states of matter
5 states of matter Flowchart of matter
Flowchart of matter Mind map on states of matter
Mind map on states of matter Phases of matter foldable
Phases of matter foldable States of matter: basics
States of matter: basics Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 Solid to gas
Solid to gas States of matter jeopardy
States of matter jeopardy States of matter thermal energy
States of matter thermal energy The fundamental difference between states of matter is the
The fundamental difference between states of matter is the Jeopardy states of matter
Jeopardy states of matter