Solutions Edward Wen Outline Composition of solution solvent

















- Slides: 48

Solutions Edward Wen

Outline • Composition of solution: solvent vs. solute • Factors affecting Solubility • Concentration of solution: Definition & Calculation ü Mass% ü Molarity* • Solution Stoichiometry 2

Solution • Homogeneous mixtures ü composition may vary from one sample to another ü appears to be one substance, though contains multiple materials • most Homogeneous materials are actually Solutions ü Gas state: common air ü Liquid: Gasoline (dozens of compounds), Soda water (sugar or asparatame, CO 2, citric acid, fructose) ü Solid: Alloy such as brass 3

Solutions: Solute + Solvent • Solute: the dissolved substance. ü Example: Sugar and CO 2 gas in Soda üseems to “disappear” ü“takes on the state” of the solvent • Solvent: the substance solute dissolves in. ü Example: Water in Soda üdoes not appear to change state • Aqueous solutions: solutions in which the solvent is water. 4

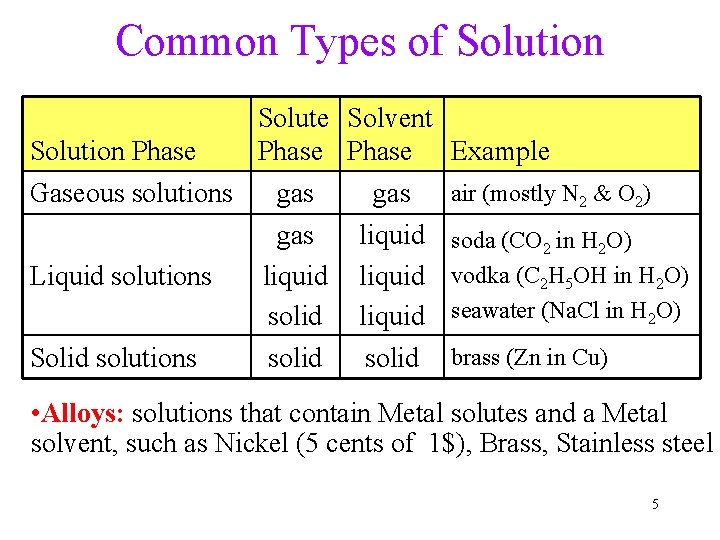
Common Types of Solution Solute Solvent Solution Phase Example Gaseous solutions gas air (mostly N 2 & O 2) gas liquid soda (CO 2 in H 2 O) liquid vodka (C 2 H 5 OH in H 2 O) Liquid solutions solid liquid seawater (Na. Cl in H 2 O) Solid solutions solid brass (Zn in Cu) • Alloys: solutions that contain Metal solutes and a Metal solvent, such as Nickel (5 cents of 1$), Brass, Stainless steel 5

How Soluble? Solubility • Soluble: when one substance (solute) dissolves in another (solvent) Homogeneous üSalt and Sugar are soluble in water: Saline and Soda üAcetic acid (HC 2 H 3 O 2) in water: Vinegar üOxygen gas in Nitrogen gas: Air • Insoluble: when one substance does not dissolve in another Heterogeneous üOil is insoluble in water: Italian salad dressing 6

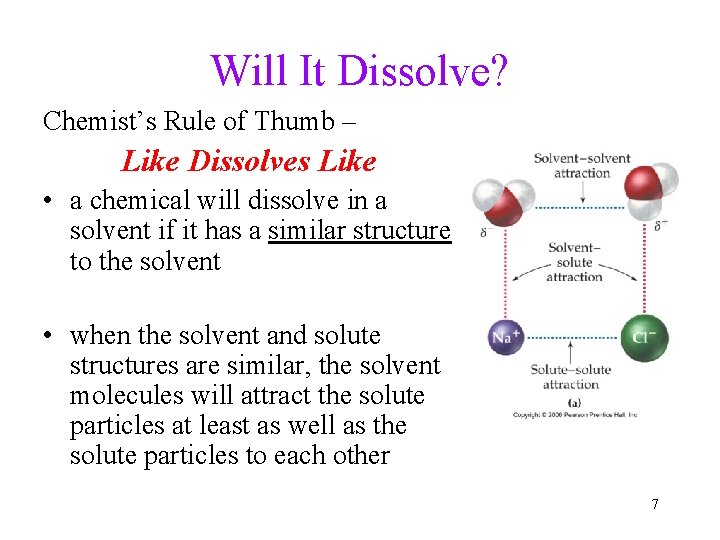
Will It Dissolve? Chemist’s Rule of Thumb – Like Dissolves Like • a chemical will dissolve in a solvent if it has a similar structure to the solvent • when the solvent and solute structures are similar, the solvent molecules will attract the solute particles at least as well as the solute particles to each other 7

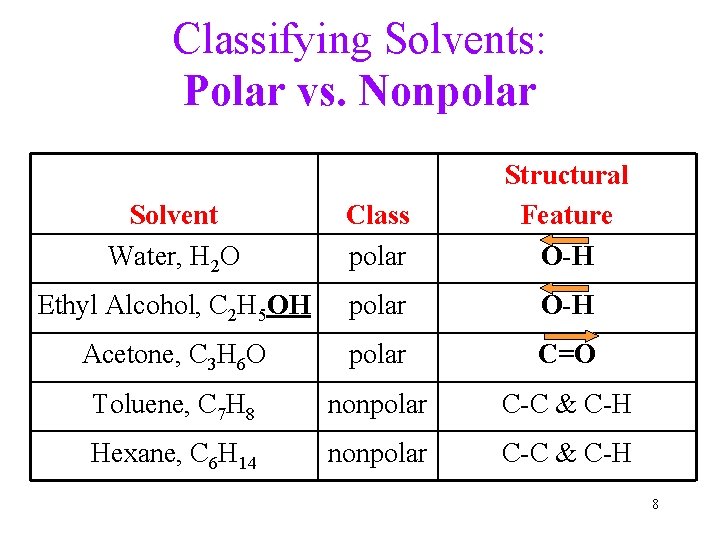
Classifying Solvents: Polar vs. Nonpolar Solvent Water, H 2 O Class polar Structural Feature O-H Ethyl Alcohol, C 2 H 5 OH polar O-H Acetone, C 3 H 6 O polar C=O Toluene, C 7 H 8 nonpolar C-C & C-H Hexane, C 6 H 14 nonpolar C-C & C-H 8

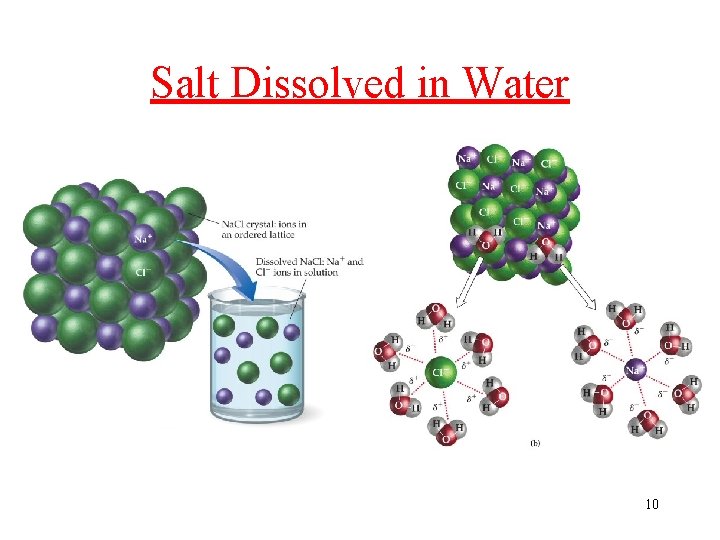
Solubility in Water, A Polar Solvent? • Ionic compound (Yes): Ions are attracted to polar Water. Salt Na. Cl dissolve in water • Polar molecules (Yes): attracted to polar solvents ü table Sugar, Alcohol, glucose • Nonpolar molecules are NOT attracted to Water ü b-carotene, (C 40 H 56), is not water soluble; it dissolves in fatty (nonpolar) tissues Those molecules with both polar and nonpolar structures: depends on structural features in the molecule 9

Salt Dissolved in Water 10

Solubility • Definition: the maximum amount of solute that can be dissolved in a given amount of solvent ü Example, at room temperature, 100. g water can dissolve 200 g sugar. Solubility = 200 g sugar/100 g water. • Usually a limit to the solubility of one substance in another ü Exceptions: gases are always soluble in each other ü two liquids that are mutually soluble are said to be miscible Øalcohol and water are miscible 11

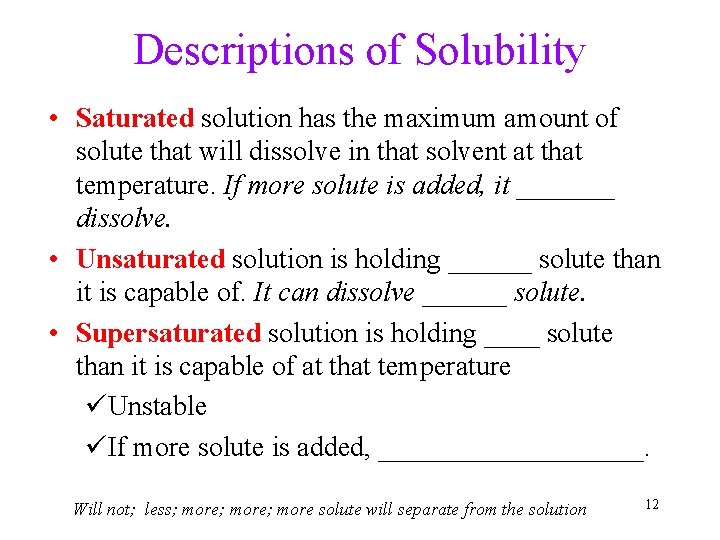
Descriptions of Solubility • Saturated solution has the maximum amount of solute that will dissolve in that solvent at that temperature. If more solute is added, it _______ dissolve. • Unsaturated solution is holding ______ solute than it is capable of. It can dissolve ______ solute. • Supersaturated solution is holding ____ solute than it is capable of at that temperature üUnstable üIf more solute is added, __________. Will not; less; more; more solute will separate from the solution 12

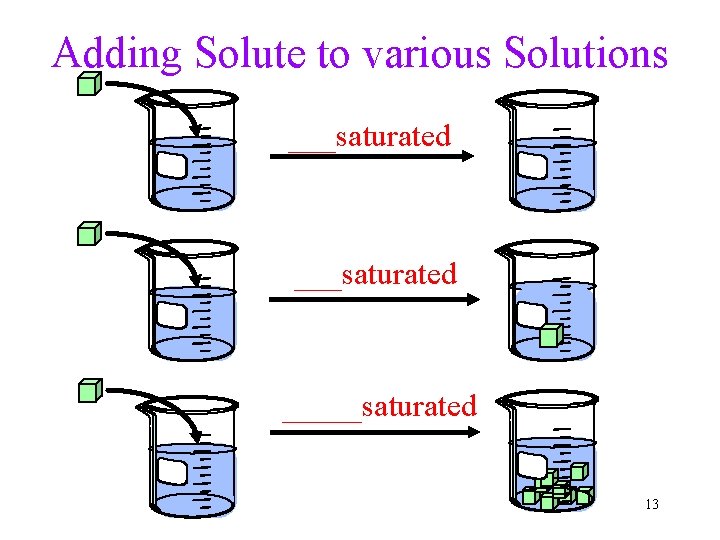
Adding Solute to various Solutions ___saturated _____saturated 13

Supersaturated Solution is unstable A supersaturated solution has more dissolved solute than the solvent can hold, not stable. When disturbed, all the solute above the saturation level will separate. Online demo: A grain of sodium acetate crystal is placed in the middle of supersatured sodium acetate solution. 14

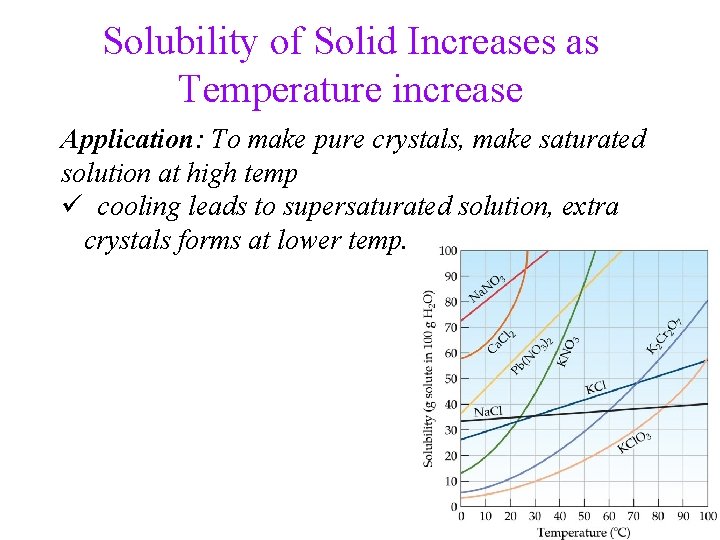
Solubility of Solid Increases as Temperature increase Application: To make pure crystals, make saturated solution at high temp ü cooling leads to supersaturated solution, extra crystals forms at lower temp. 15

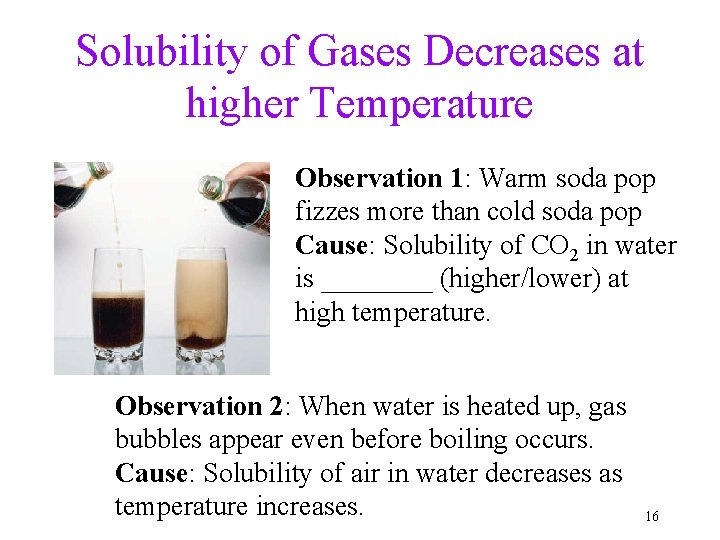
Solubility of Gases Decreases at higher Temperature Observation 1: Warm soda pop fizzes more than cold soda pop Cause: Solubility of CO 2 in water is ____ (higher/lower) at high temperature. Observation 2: When water is heated up, gas bubbles appear even before boiling occurs. Cause: Solubility of air in water decreases as temperature increases. 16

Solubility of Gas depends on Pressure • higher pressure = higher solubility • CO 2 is dissolved under Pressure into bottled/canned soda 17

Solution Concentration Descriptions • Diluted solutions have low solute concentrations. Soda drink from the soda fountain. • Concentrated solutions have high solute concentrations. Syrup in the storage tank in the soda fountain. 18

Concentrations – Quantitative Descriptions of Solutions • Solutions have variable composition. Salt vs. Water in Seawater • To describe a solution accurately, you need to describe the components and their relative amounts • Concentration = amount of solute in a given amount of solution ü Seawater: Salt concentration 3. 4% ü Dead Sea: Salt concentration 30% ü Vinegar: Acetic acid concentration 5% 19

Mass Percent (%) • mass of solute (gram) in every 100 gram of solution ü if a solution is 0. 9% by mass, then there are 0. 9 grams of solute in every 100 grams of solution • Mass of solution = Mass of solute + Mass of solvent Mass% = _________ x _____ 20

Example: Calculate the mass percent of a solution containing 27. 5 g of ethanol (C 2 H 6 O) and 175 m. L of H 2 O (assume the density of H 2 O is 1. 00 g/m. L) Information Given: 27. 5 g C 2 H 6 O; 175 m. L H 2 O Find: % by mass Mass of Solvent = 175 g Mass of Solution = 202. 5 g Mass of Solute = 27. 5 g Mass% = 13. 6 21

Using Concentrations as Conversion Factors • concentrations show the relationship between the amount of solute and the amount of solvent ü 12% by mass sugar(aq) means 12 g sugar 100 g solution • The concentration can then be used to convert the amount of solute into the amount of solution, or visa versa 22

Example: A soft drink contains 11. 5% sucrose (C 12 H 22 O 11) by mass. What volume of soft drink in milliliters contains 85. 2 g of sucrose? (assume the density is 1. 00 g/m. L) Information Given: 85. 2 g C 12 H 22 O 11 Find: m. L sol’n V = 741 m. L 23

Preparing a Solution • What we need to know: Amount of solution & Concentration of solution • Calculate the mass of solute needed üstart with amount of solution üuse concentration as a conversion factor ü 5% by mass solute Þ 5 g solute 100 g solution 24

Preparing a Solution by Mass% Example - How would you prepare 250. 0 g of 5. 00% by mass glucose solution (normal glucose)? 12. 5 g of glucose 237. 5 g of water 25

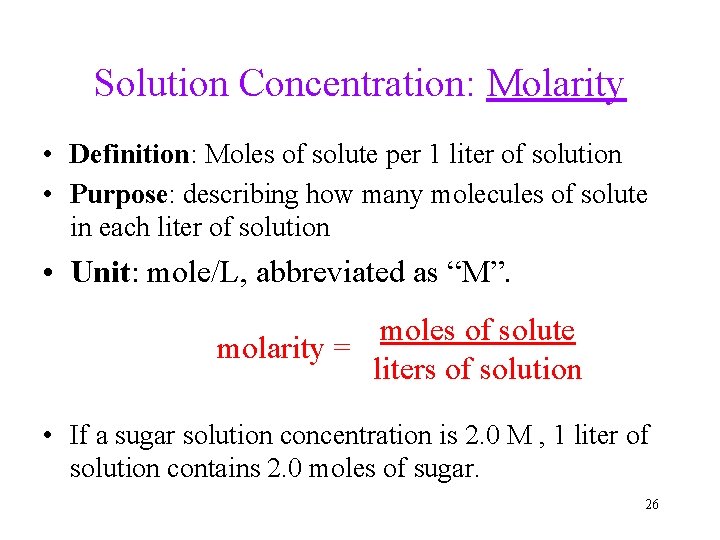
Solution Concentration: Molarity • Definition: Moles of solute per 1 liter of solution • Purpose: describing how many molecules of solute in each liter of solution • Unit: mole/L, abbreviated as “M”. moles of solute molarity = liters of solution • If a sugar solution concentration is 2. 0 M , 1 liter of solution contains 2. 0 moles of sugar. 26

Why Molarity? Many reagents used in chemistry, even many biology labs, are in the form of solution. Molarity concentration of solution is particularly important and useful because: • Easy to use: To obtain given amount (mole) of reagent, just calculate the volume of solution to be used: • Easy to prepare a solution to a given molarity 27

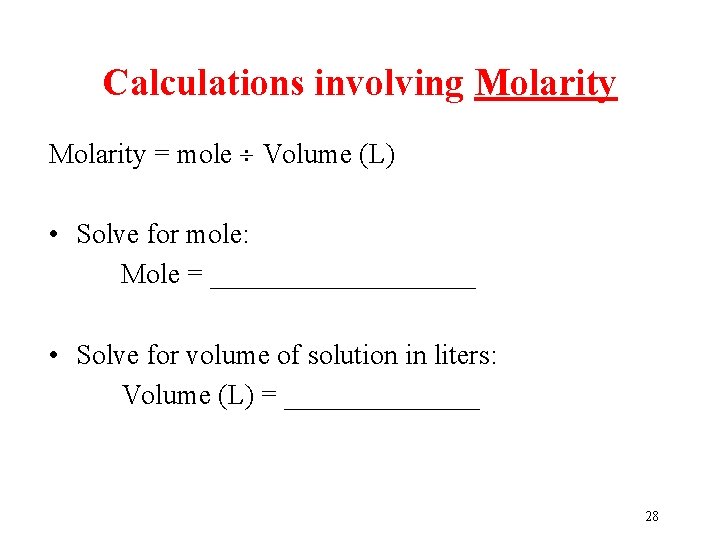
Calculations involving Molarity = mole Volume (L) • Solve for mole: Mole = __________ • Solve for volume of solution in liters: Volume (L) = _______ 28

Example – How to prepare 500 m. L of 0. 020 M Na. Cl solution? Part A. Calculate the mass Na. Cl needed: 0. 010 mol Na. Cl 0. 58 g Na. Cl 29

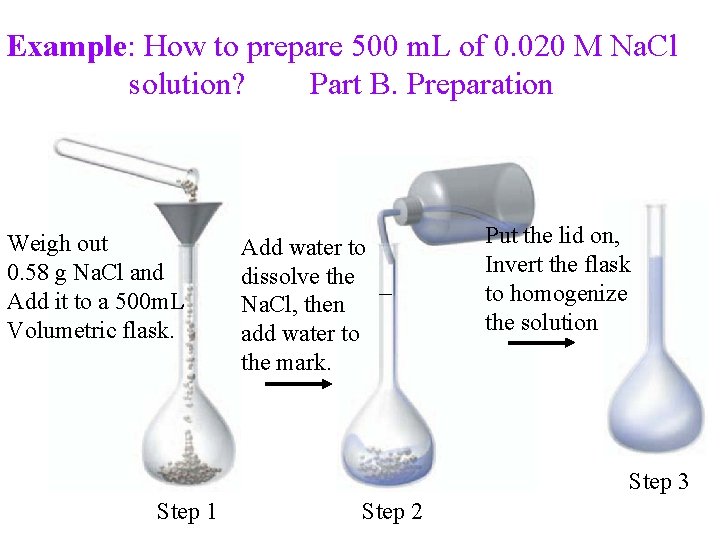
Example: How to prepare 500 m. L of 0. 020 M Na. Cl solution? Part B. Preparation Weigh out 0. 58 g Na. Cl and Add it to a 500 m. L Volumetric flask. Add water to dissolve the Na. Cl, then add water to the mark. Put the lid on, Invert the flask to homogenize the solution Step 3 Step 1 Step 2

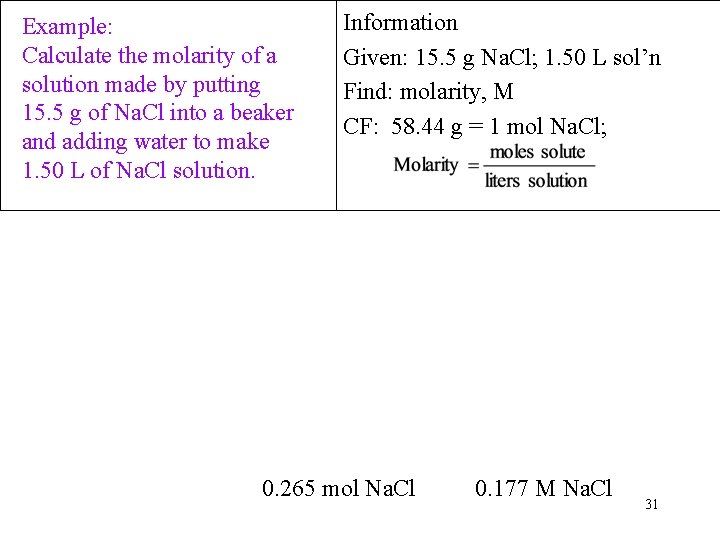
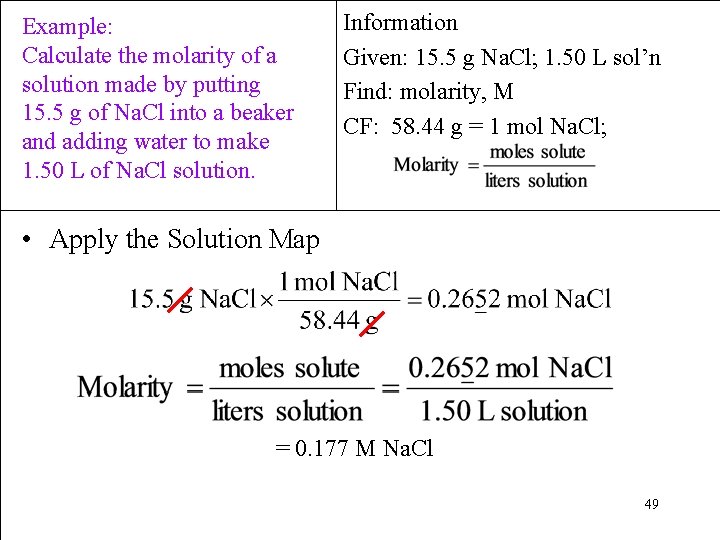
Example: Calculate the molarity of a solution made by putting 15. 5 g of Na. Cl into a beaker and adding water to make 1. 50 L of Na. Cl solution. Information Given: 15. 5 g Na. Cl; 1. 50 L sol’n Find: molarity, M CF: 58. 44 g = 1 mol Na. Cl; 0. 265 mol Na. Cl 0. 177 M Na. Cl 31

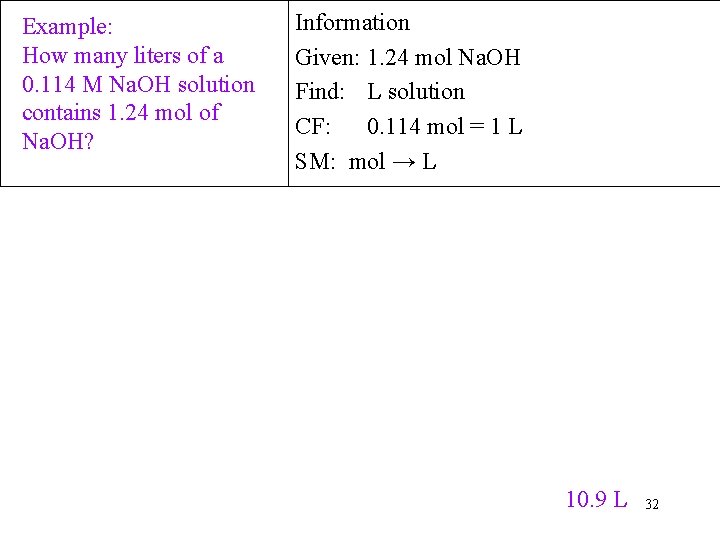
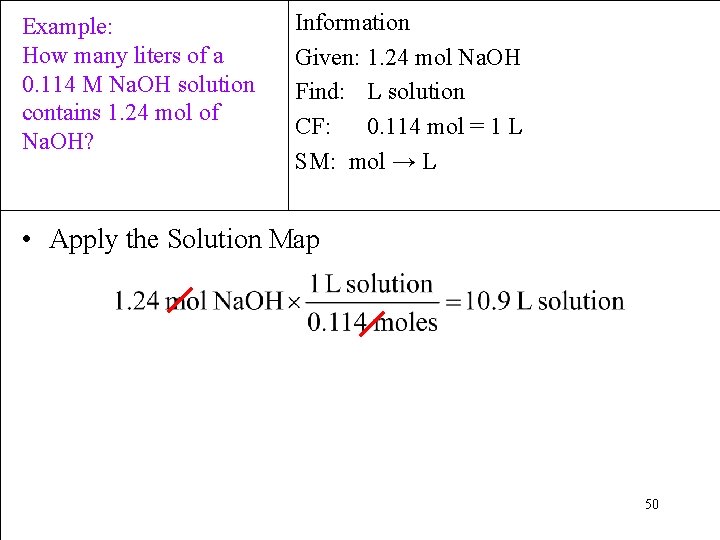
Example: How many liters of a 0. 114 M Na. OH solution contains 1. 24 mol of Na. OH? Information Given: 1. 24 mol Na. OH Find: L solution CF: 0. 114 mol = 1 L SM: mol → L 10. 9 L 32

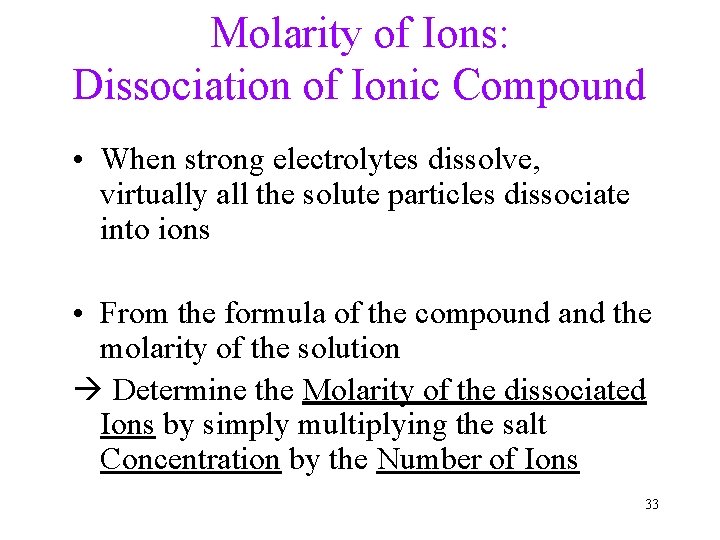
Molarity of Ions: Dissociation of Ionic Compound • When strong electrolytes dissolve, virtually all the solute particles dissociate into ions • From the formula of the compound and the molarity of the solution Determine the Molarity of the dissociated Ions by simply multiplying the salt Concentration by the Number of Ions 33

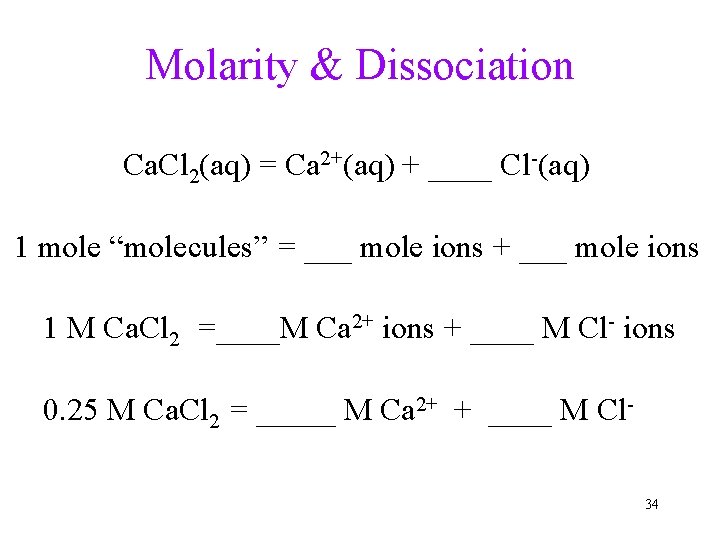
Molarity & Dissociation Ca. Cl 2(aq) = Ca 2+(aq) + ____ Cl-(aq) 1 mole “molecules” = ___ mole ions + ___ mole ions 1 M Ca. Cl 2 =____M Ca 2+ ions + ____ M Cl- ions 0. 25 M Ca. Cl 2 = _____ M Ca 2+ + ____ M Cl 34

Making a Solution by Dilution Example: A student added 1. 00 L water to 2. 00 L 1. 00 M HCl. The final volume became 3. 00 L. mole HCl before mixing = M 1 x V 1 = 2. 00 mole = mole HCl after mixing more water. • When mixing more solvent into a solution, the volume of final solution is greater than the original solution • The mole of solute remains the same before and after mixing more solvent For dilution, mole solute = M 1 x V 1 = M 2 x V 2

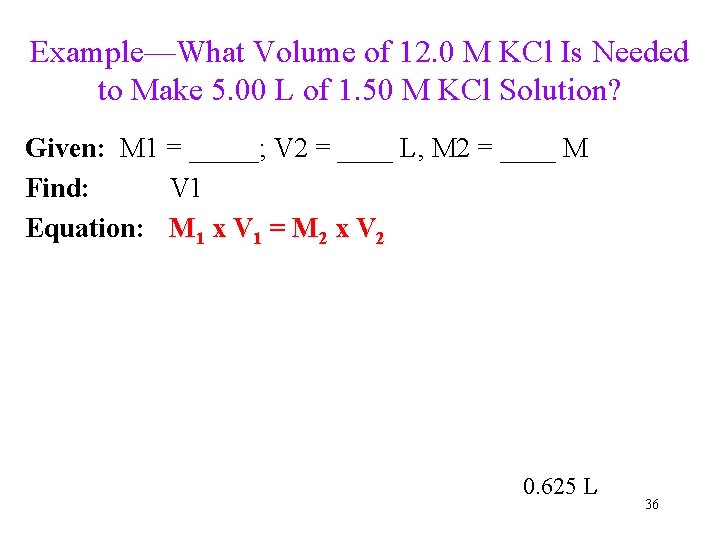
Example—What Volume of 12. 0 M KCl Is Needed to Make 5. 00 L of 1. 50 M KCl Solution? Given: M 1 = _____; V 2 = ____ L, M 2 = ____ M Find: V 1 Equation: M 1 x V 1 = M 2 x V 2 0. 625 L 36

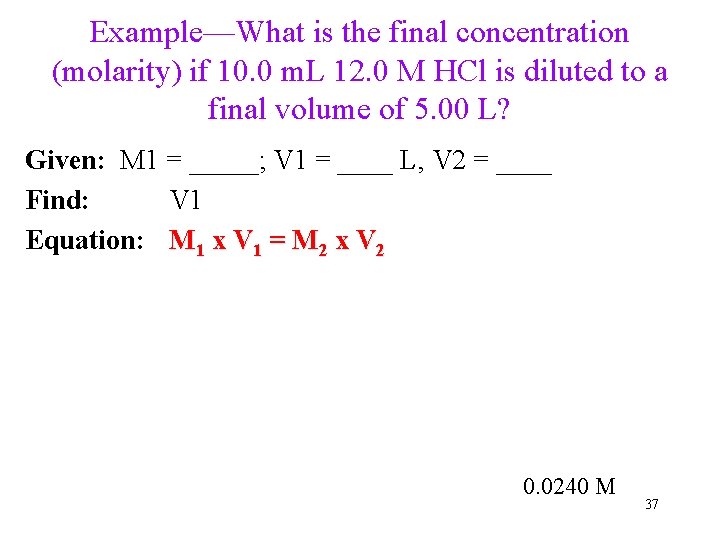
Example—What is the final concentration (molarity) if 10. 0 m. L 12. 0 M HCl is diluted to a final volume of 5. 00 L? Given: M 1 = _____; V 1 = ____ L, V 2 = ____ Find: V 1 Equation: M 1 x V 1 = M 2 x V 2 0. 0240 M 37

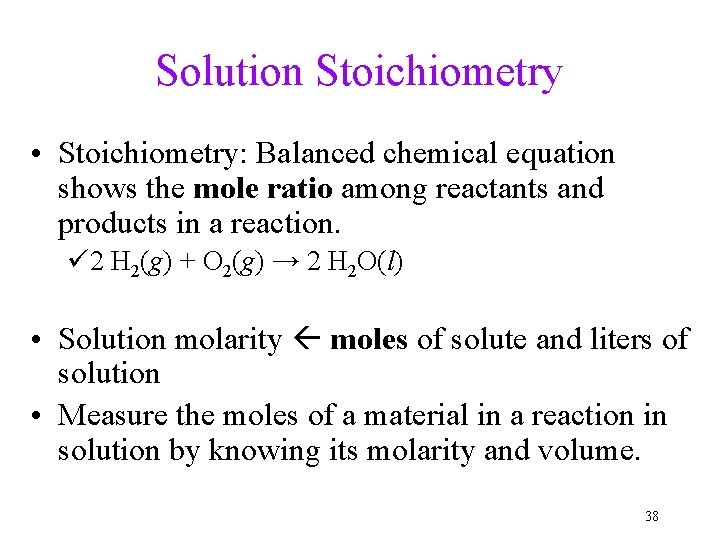
Solution Stoichiometry • Stoichiometry: Balanced chemical equation shows the mole ratio among reactants and products in a reaction. ü 2 H 2(g) + O 2(g) → 2 H 2 O(l) • Solution molarity moles of solute and liters of solution • Measure the moles of a material in a reaction in solution by knowing its molarity and volume. 38

Full solution for Examples 41

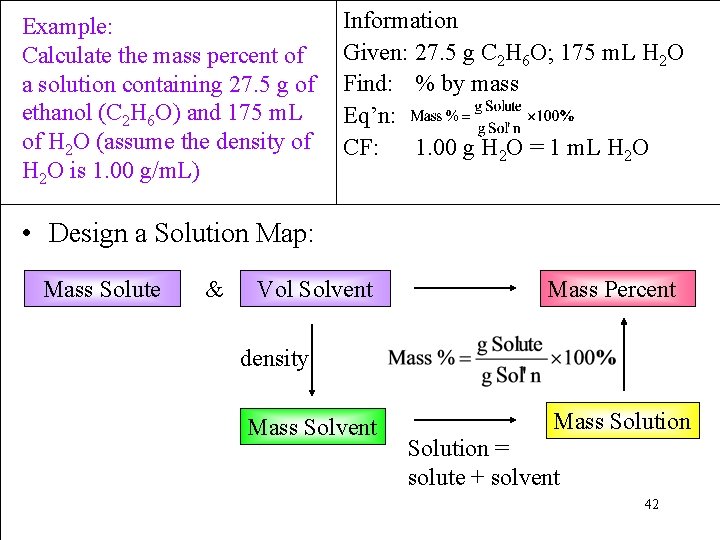
Example: Calculate the mass percent of a solution containing 27. 5 g of ethanol (C 2 H 6 O) and 175 m. L of H 2 O (assume the density of H 2 O is 1. 00 g/m. L) Information Given: 27. 5 g C 2 H 6 O; 175 m. L H 2 O Find: % by mass Eq’n: CF: 1. 00 g H 2 O = 1 m. L H 2 O • Design a Solution Map: Mass Solute & Vol Solvent Mass Percent density Mass Solvent Mass Solution = solute + solvent 42

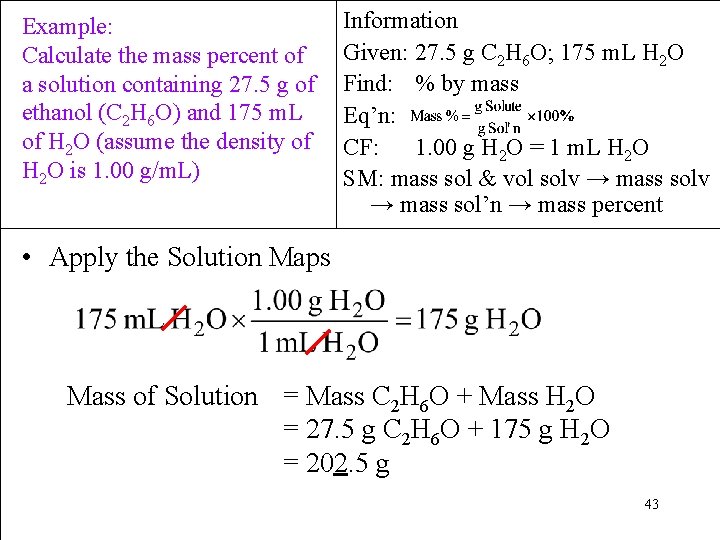
Example: Calculate the mass percent of a solution containing 27. 5 g of ethanol (C 2 H 6 O) and 175 m. L of H 2 O (assume the density of H 2 O is 1. 00 g/m. L) Information Given: 27. 5 g C 2 H 6 O; 175 m. L H 2 O Find: % by mass Eq’n: CF: 1. 00 g H 2 O = 1 m. L H 2 O SM: mass sol & vol solv → mass sol’n → mass percent • Apply the Solution Maps Mass of Solution = Mass C 2 H 6 O + Mass H 2 O = 27. 5 g C 2 H 6 O + 175 g H 2 O = 202. 5 g 43

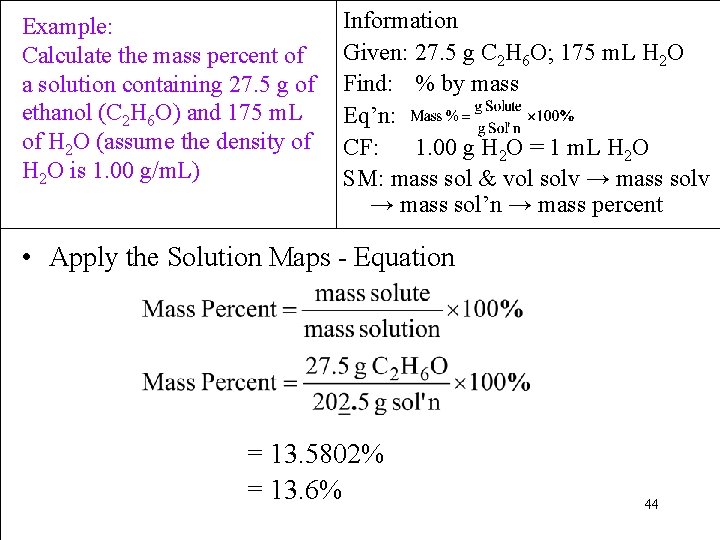
Example: Calculate the mass percent of a solution containing 27. 5 g of ethanol (C 2 H 6 O) and 175 m. L of H 2 O (assume the density of H 2 O is 1. 00 g/m. L) Information Given: 27. 5 g C 2 H 6 O; 175 m. L H 2 O Find: % by mass Eq’n: CF: 1. 00 g H 2 O = 1 m. L H 2 O SM: mass sol & vol solv → mass sol’n → mass percent • Apply the Solution Maps - Equation = 13. 5802% = 13. 6% 44

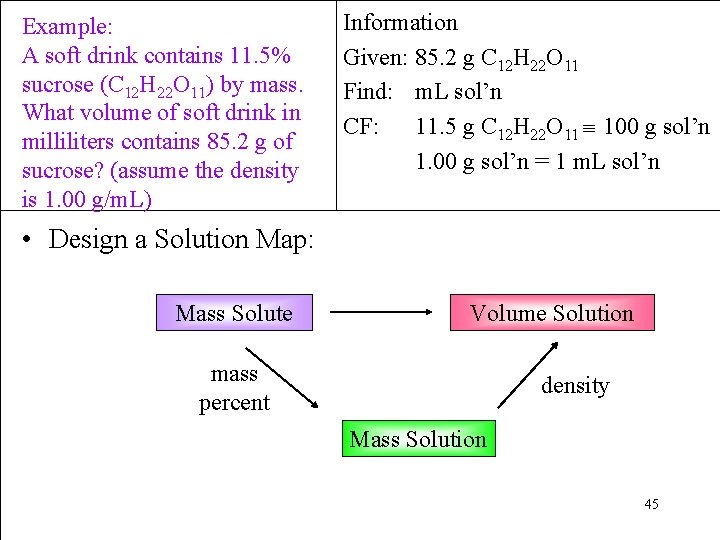
Example: A soft drink contains 11. 5% sucrose (C 12 H 22 O 11) by mass. What volume of soft drink in milliliters contains 85. 2 g of sucrose? (assume the density is 1. 00 g/m. L) Information Given: 85. 2 g C 12 H 22 O 11 Find: m. L sol’n CF: 11. 5 g C 12 H 22 O 11 100 g sol’n 1. 00 g sol’n = 1 m. L sol’n • Design a Solution Map: Mass Solute Volume Solution mass percent density Mass Solution 45

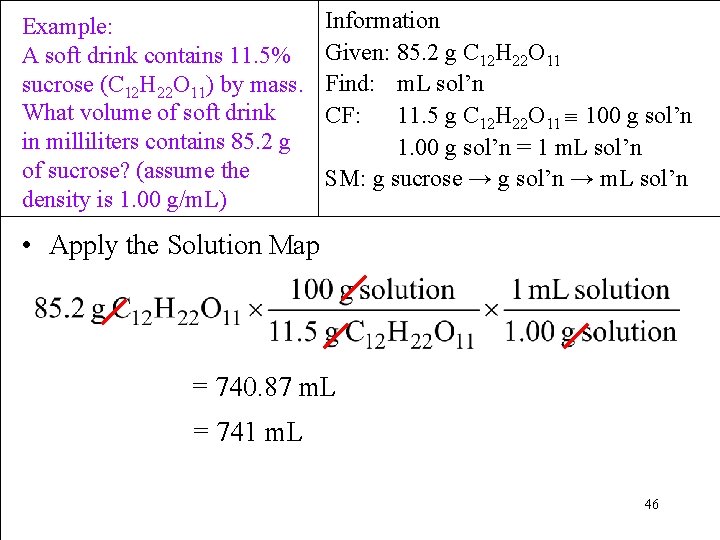
Example: A soft drink contains 11. 5% sucrose (C 12 H 22 O 11) by mass. What volume of soft drink in milliliters contains 85. 2 g of sucrose? (assume the density is 1. 00 g/m. L) Information Given: 85. 2 g C 12 H 22 O 11 Find: m. L sol’n CF: 11. 5 g C 12 H 22 O 11 100 g sol’n 1. 00 g sol’n = 1 m. L sol’n SM: g sucrose → g sol’n → m. L sol’n • Apply the Solution Map = 740. 87 m. L = 741 m. L 46

Preparing a Solution by Mass% Example - How would you prepare 250. 0 g of 5. 00% by mass glucose solution (normal glucose)? Dissolve 12. 5 g of glucose in 237. 5 g of water to give the solution 47

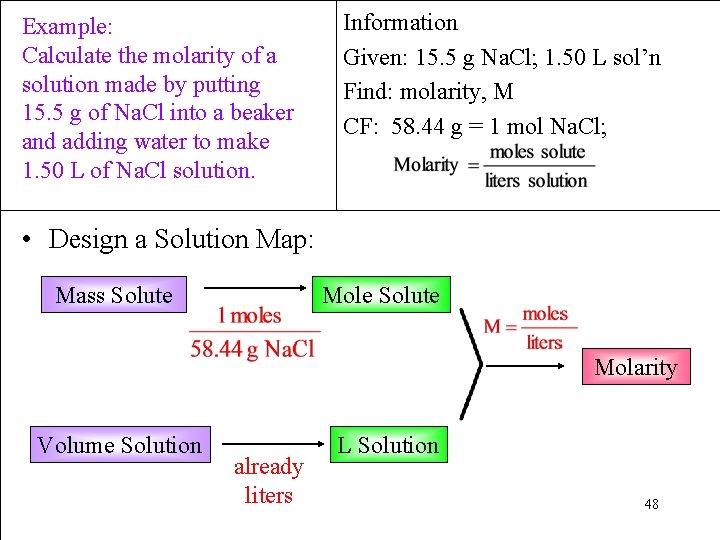
Example: Calculate the molarity of a solution made by putting 15. 5 g of Na. Cl into a beaker and adding water to make 1. 50 L of Na. Cl solution. Information Given: 15. 5 g Na. Cl; 1. 50 L sol’n Find: molarity, M CF: 58. 44 g = 1 mol Na. Cl; • Design a Solution Map: Mass Solute Mole Solute Molarity Volume Solution already liters L Solution 48

Example: Calculate the molarity of a solution made by putting 15. 5 g of Na. Cl into a beaker and adding water to make 1. 50 L of Na. Cl solution. Information Given: 15. 5 g Na. Cl; 1. 50 L sol’n Find: molarity, M CF: 58. 44 g = 1 mol Na. Cl; • Apply the Solution Map = 0. 177 M Na. Cl 49

Example: How many liters of a 0. 114 M Na. OH solution contains 1. 24 mol of Na. OH? Information Given: 1. 24 mol Na. OH Find: L solution CF: 0. 114 mol = 1 L SM: mol → L • Apply the Solution Map 50