Solutions AP Chemistry Solution A homogeneous mixture of









- Slides: 19

Solutions AP Chemistry

Solution: A homogeneous mixture of solute particles dissolved in a solvent. The Solvent The solvent retains its phase. The solvent is present in greater amount (if the same phase as the solute).

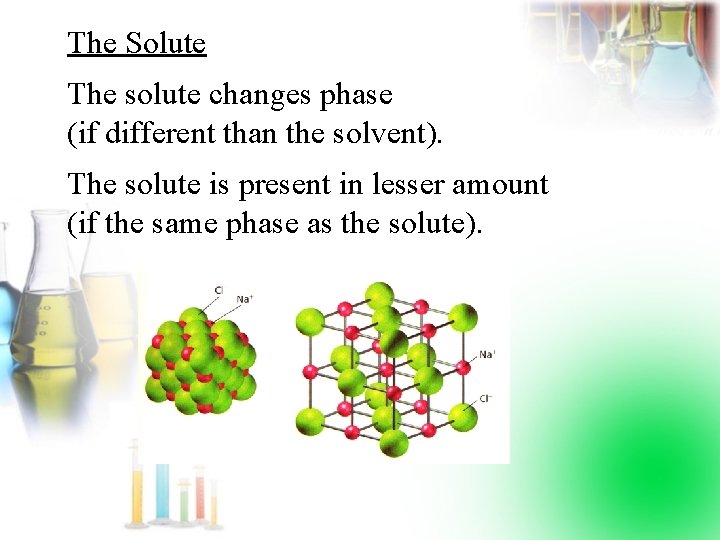
The Solute The solute changes phase (if different than the solvent). The solute is present in lesser amount (if the same phase as the solute).

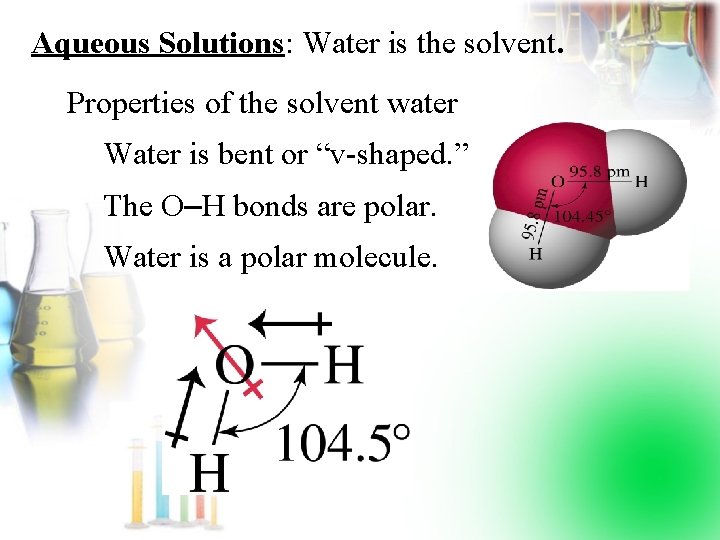
Aqueous Solutions: Water is the solvent. Properties of the solvent water Water is bent or “v-shaped. ” The O–H bonds are polar. Water is a polar molecule.

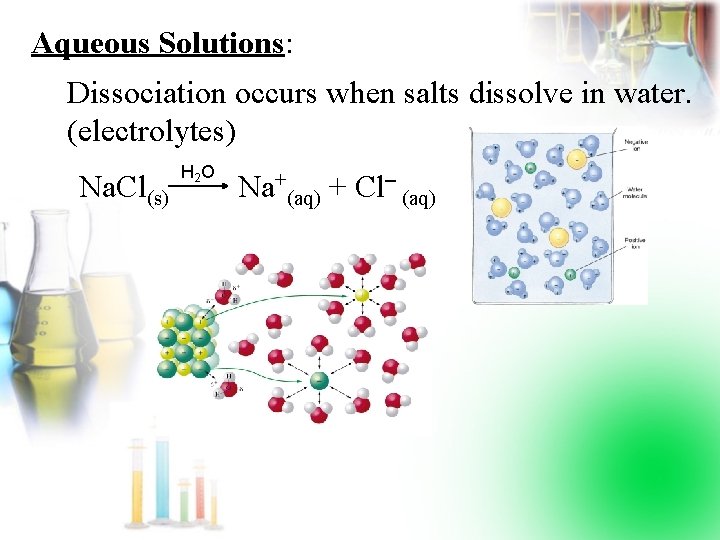
Aqueous Solutions: Dissociation occurs when salts dissolve in water. (electrolytes) Na. Cl(s) H 2 O Na+(aq) + Cl (aq)

Aqueous Solutions: An electrolyte is a substance that conducts electricity when dissolved in water. Only dissolution occurs when neutral, covalent compounds dissolve in water. No dissociation. (non-electrolytes)

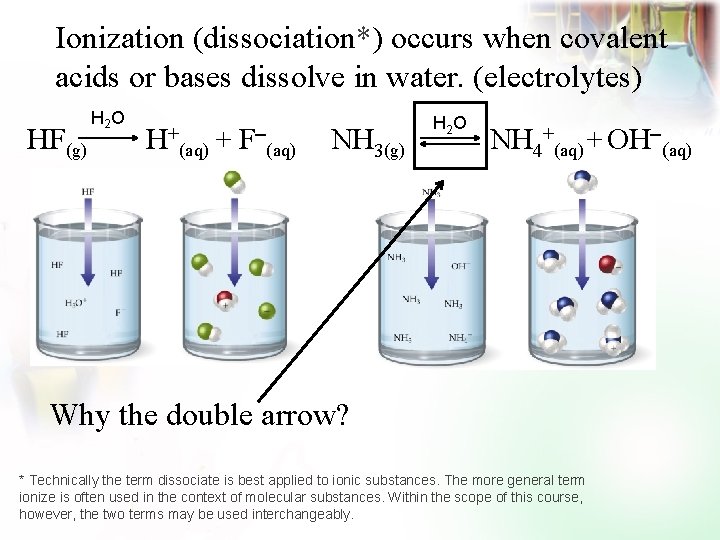
Ionization (dissociation*) occurs when covalent acids or bases dissolve in water. (electrolytes) HF(g) H 2 O H+ (aq) + F (aq) NH 3(g) H 2 O NH 4+(aq) + OH (aq) Why the double arrow? * Technically the term dissociate is best applied to ionic substances. The more general term ionize is often used in the context of molecular substances. Within the scope of this course, however, the two terms may be used interchangeably.

Identify the solutes on the following slides as strong electrolytes, weak electrolytes, or neither? Explain why. .

Hydrogen chloride Answer: Strong electrolyte Explanation: Strong acids ionize (dissociate) completely or nearly completely*. HCl(g) H 2 O H+(aq) + Cl (aq) * Technically the term dissociate is best applied to ionic substances. The more general term ionize is often used in the context of molecular substances. Within the scope of this course, however, the two terms may be used interchangeably. Further, the idea of “complete” or “nearly complete” dissociation applies only to dilute solutions – those less than 1. 0 M.

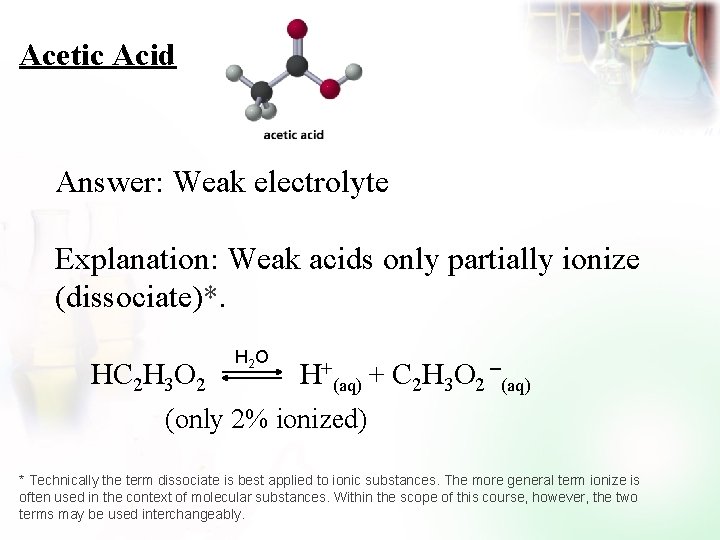
Acetic Acid Answer: Weak electrolyte Explanation: Weak acids only partially ionize (dissociate)*. HC 2 H 3 O 2 H 2 O H+(aq) + C 2 H 3 O 2 (aq) (only 2% ionized) * Technically the term dissociate is best applied to ionic substances. The more general term ionize is often used in the context of molecular substances. Within the scope of this course, however, the two terms may be used interchangeably.

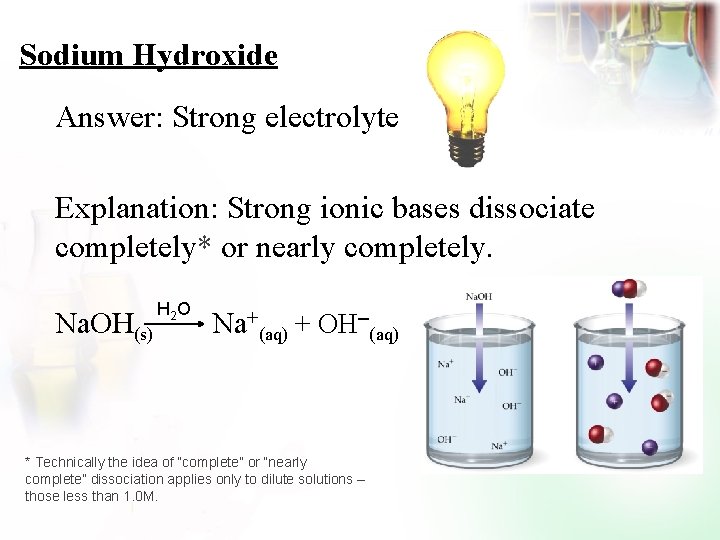
Sodium Hydroxide Answer: Strong electrolyte Explanation: Strong ionic bases dissociate completely* or nearly completely. Na. OH(s) H 2 O Na+(aq) + OH (aq) * Technically the idea of “complete” or “nearly complete” dissociation applies only to dilute solutions – those less than 1. 0 M.

Ammonia Answer: Weak electrolyte Explanation: Weak bases only partially ionize (dissociate*). NH 3(g) H 2 O NH 4+(aq) + OH (aq) (only 1 to 5% ionized) * Technically the term dissociate is best applied to ionic substances. The more general term ionize is often used in the context of molecular substances. Within the scope of this course, however, the two terms may be used interchangeably.

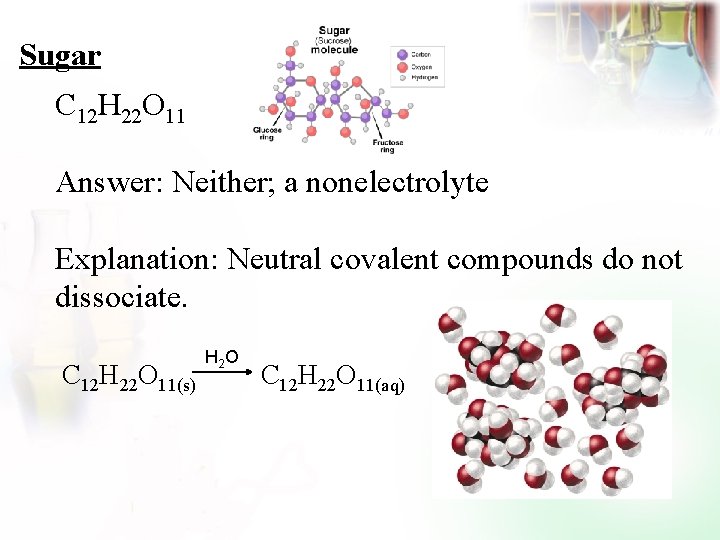
Sugar C 12 H 22 O 11 Answer: Neither; a nonelectrolyte Explanation: Neutral covalent compounds do not dissociate. C 12 H 22 O 11(s) H 2 O C 12 H 22 O 11(aq)

Aluminum Hydroxide Al(OH)3 Answer: Strong, but impractical; why the later? Explanation: Aluminum hydroxide is an insoluble solid. Only the tiny bit that dissolves would completely dissociate.

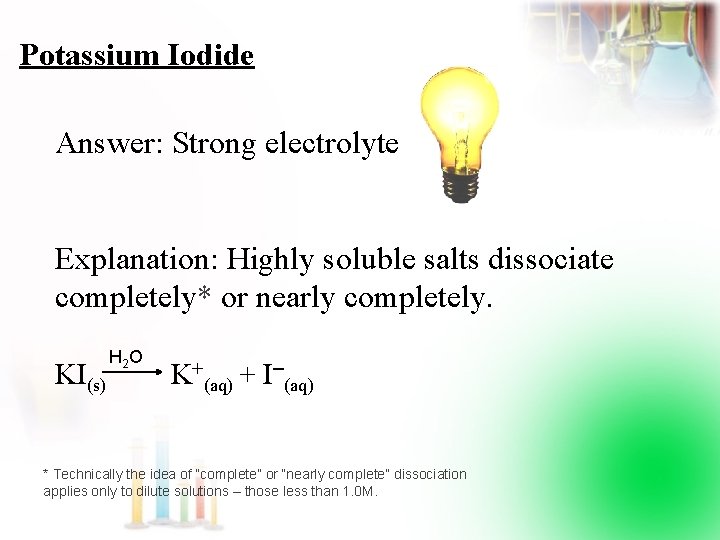
Potassium Iodide Answer: Strong electrolyte Explanation: Highly soluble salts dissociate completely* or nearly completely. KI(s) H 2 O K+(aq) + I (aq) * Technically the idea of “complete” or “nearly complete” dissociation applies only to dilute solutions – those less than 1. 0 M.

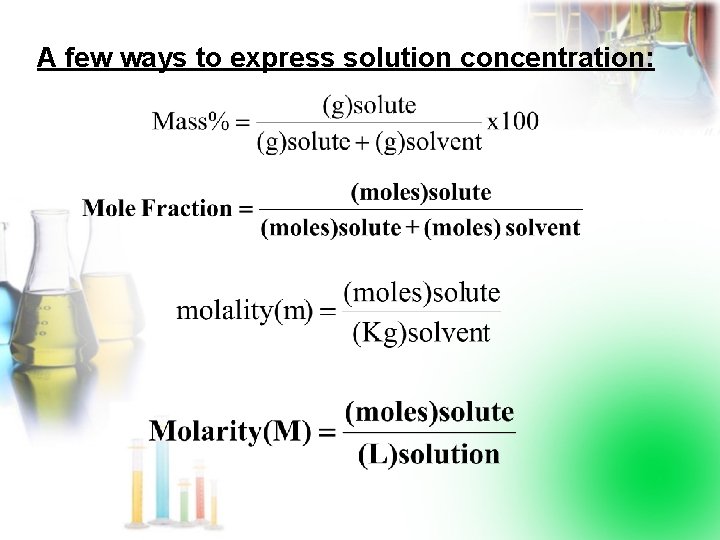
A few ways to express solution concentration:

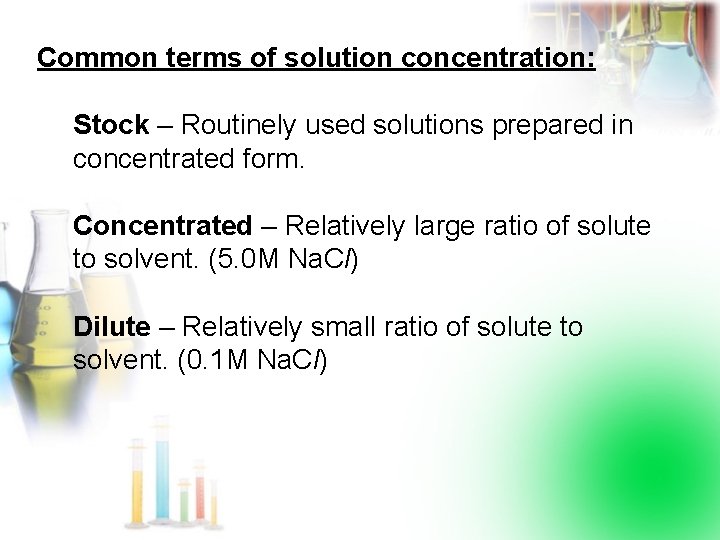
Common terms of solution concentration: Stock – Routinely used solutions prepared in concentrated form. Concentrated – Relatively large ratio of solute to solvent. (5. 0 M Na. Cl) Dilute – Relatively small ratio of solute to solvent. (0. 1 M Na. Cl)

Diluting stock or concentrated solutions: Use Mc. Vc=Md. Vd Solve for the concentrate value, add this to a volumetric flask and top off with water. AP Exam Free Answer Question Year 2003 Exam. Free Answer Question. Part (a) only.

AP Exam Multiple Choice Question: 1989, #43 (46% of students got this correct)