Sixth Annual Meeting March 12 2012 The Key

























- Slides: 45

Sixth Annual Meeting, March 12, 2012 The Key to Success: INTERMACS Hospitals • Site Utilization of INTERMACS Data and Reports for local QI • Quality Assurance and data quality • Evaluation of Site Data: Audits, l M a u 2 n Complete Enrollment, Complete Data, n 01 A 2 S h etc. C c r A E T IN a M M R g in t ee

Sixth Annual Meeting, March 12, 2012 The Key to Success: INTERMACS Hospitals! g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

Sixth Annual Meeting, March 12, 2012 The Key to Success: INTERMACS Hospitals • Site Utilization of INTERMACS Data and Reports for local QI Naftel g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

Hospitals • What services do the hospitals receive for their participation fee? • Services • Meets CMS/Joint Commission requirement for Destination Therapy Certification • Meets FDA required submission of Medical Device Reports (MDRs) by hospitals • Provides clinical summaries of patients • Provides quality assurance reports • Provides electronic data transfer g n i t • Provides standardized datasets e e M • Provides benchmarking l a u • Provides training and continuing education units nn 012 A 2 S h C c r A a M M R E T IN

Hospitals (Continued) • What benefits do the hospitals receive for their participation fee? • Benefits • Fulfills CMS DT Certification requirement • Become part of the national dialogue on the evaluation and evolution of MCSDs • Invited to participate in the INTERMACS Annual Meeting • Invited to join the INTERMACS Committees g • Coordinators Council and other committees it n • Select Hospital Administrators will have the opportunity ee l M to serve on the Business Advisory Committee a u 2 n n 01 A 2 S h C c r A a M M R E T IN

Sixth Annual Meeting, March 12, 2012 The Key to Success: INTERMACS Hospitals • Quality Assurance and data quality Naftel g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

Fifth Annual Meeting, April 12, 2011 Hospital Perspective – Deliverables Coordinators Clinical Team Quality Assurance Officer Office of Risk Management Financial Officers / Administrators Office of Accreditation Researchers g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

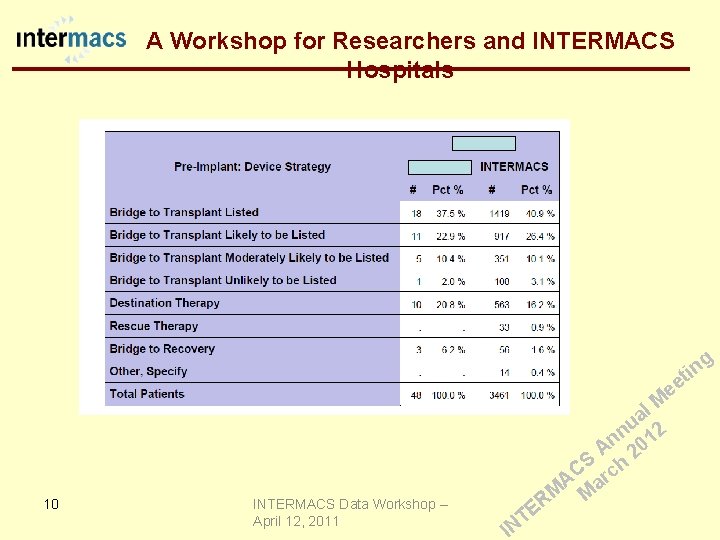
A Workshop for Researchers and INTERMACS Hospitals QA Report g in t ee 8 INTERMACS Data Workshop – April 12, 2011 E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

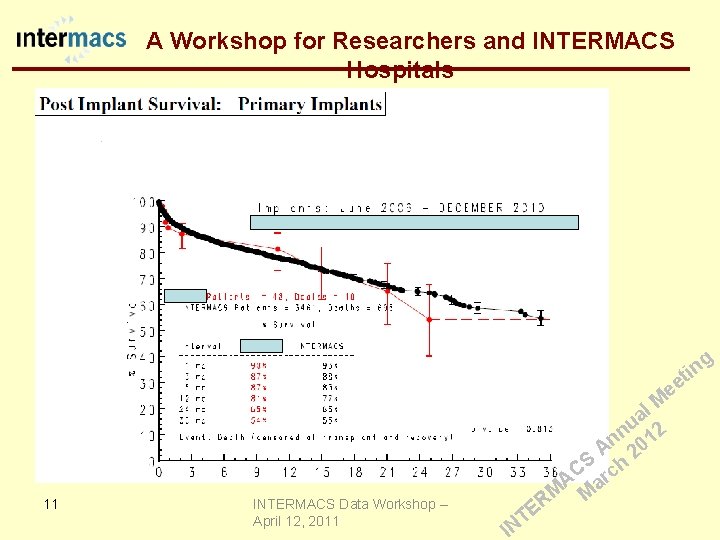
A Workshop for Researchers and INTERMACS Hospitals QA Report g in t ee 9 INTERMACS Data Workshop – April 12, 2011 E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

A Workshop for Researchers and INTERMACS Hospitals g in t ee 10 INTERMACS Data Workshop – April 12, 2011 E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

A Workshop for Researchers and INTERMACS Hospitals g in t ee 11 INTERMACS Data Workshop – April 12, 2011 E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

Sixth Annual Meeting, March 12, 2012 The Key to Success: INTERMACS Hospitals • Evaluation of Site Data: Audits, Complete Enrollment, Complete Data, etc. Naftel E T IN g l M a u 2 n n 01 A 2 S h C c r A a M M R in t ee

Sixth Annual Meeting, March 12, 2012 1. Regulatory Requirements Regulatory requirements must be met. Assessment: UNOS collects and the DCC evaluates all regulatory documents Goal: 100% of participating hospitals meet all g regulatory requirements it n Minimal Standard: 100% ee M l a u E T IN n 12 n A 20 S h C A arc RM M

Coordinator Training, March 11, 2012 133 1, 705 1, 267 1, 590 Activated Sites (currently 131) INTERMACS Personnel IRB Approvals since 2006 Informed Consent, HIPAA, Revoke Authorization, Transfer, Blood / Tissue documents 1, 490 Human Subjects Training Certificateseting e M 1, 805 Financial Disclosure / Conflict of nual 2 n 01 A Interest S h 2 E T IN AC arc RM M

Sixth Annual Meeting, March 12, 2012 2. Timely Follow-up data Assessment: will focus on submission of follow-up forms. Goal: 100% of follow-up forms submitted within 30 days of the date of expected follow-up. g Minimal Standard: 90% of follow-up forms must be it n submitted within 30 days of the date of expected follow-ee M l a up u E T IN n 12 n A 20 S h C A arc RM M

Coordinator Training Session: March 11, 2012 Overview of Data Entry Naftel g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R 16

Sixth Annual Meeting, March 12, 2012 3. All Device Implants Complete Accounting of all eligible device implants. Assessment: Matching hospital enrollment to industry counts. Goal: 100% of eligible devices enrolled. g Minimal Standard: 90% of eligible devices enrolled. it n Note 1: All eligible DT devices must be entered. ee M l a Note 2: Informed consent is a barrier to the minimal u n 12 n standard. A 20 E T IN S h C A arc RM M

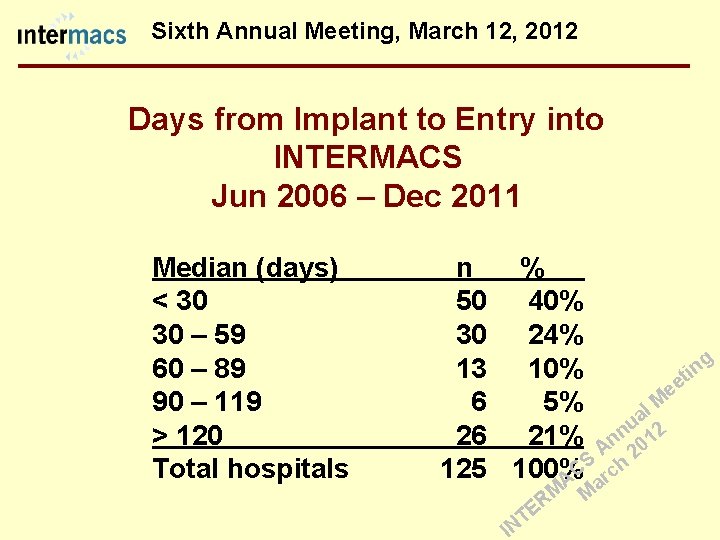
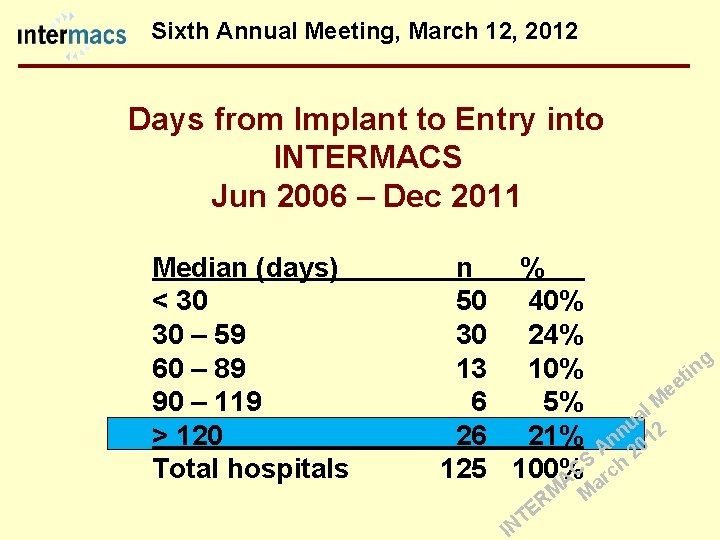
Sixth Annual Meeting, March 12, 2012 Days from Implant to Entry into INTERMACS Jun 2006 – Dec 2011 Median (days) < 30 30 – 59 60 – 89 90 – 119 > 120 Total hospitals n 50 30 13 6 26 125 % 40% 24% g 10% it n ee 5% l M a u 2 n 21% An 01 2 S h C 100% c r A a M M R E T IN

Sixth Annual Meeting, March 12, 2012 Days from Implant to Entry into INTERMACS Jun 2006 – Dec 2011 Median (days) < 30 30 – 59 60 – 89 90 – 119 > 120 Total hospitals n 50 30 13 6 26 125 % 40% 24% g 10% it n ee 5% l M a u 2 n 21% An 01 2 S h C 100% c r A a M M R E T IN

Sixth Annual Meeting, March 12, 2012 4. Completeness of data elements Assessment: The web-based application requires that all elements be addressed (either a data value entered or “not done” selected) before the form can be submitted. The proportion of captured data values will g n i t be calculated. ee l M Goal: 100% completion of data elements. a u 2 n Minimal Standard: To be determined after consultation n 01 A 2 S with HSC. C rch E T IN A a M M R

Sixth Annual Meeting, March 12, 2012 Completeness of Quality of Life Data will be addressed by Dr. Grady g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

Sixth Annual Meeting, March 12, 2012 The Key to Success: INTERMACS Hospitals • Risk adjustment: Will require an in-depth, collaborative effort among the INTERMACS Collaborators including g it n hospital representatives. The approach will be ee l M a modeled after the SRTR evaluation of post u 2 n n 01 A heart transplant survival. S h 2 E T IN AC arc RM M

Sixth Annual Meeting, March 12, 2012 Update on New INTERMACS/NIH Initiatives • Meda. MACS • Pump. KIN • Pedi. MACS • IMACS • Revive-IT g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

Sixth Annual Meeting, March 12, 2012 Update on New INTERMACS/NIH Initiatives • Meda. MACS (15 min) Stewart g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R

MEDAMACS Update 2012: Medical Arm of Mechanical Circulatory Support INTERMACS - 6 th Annual Meeting March 12, 2012 E T IN g l M a u 2 n n 01 A 2 S h C c r A a M M R in t ee

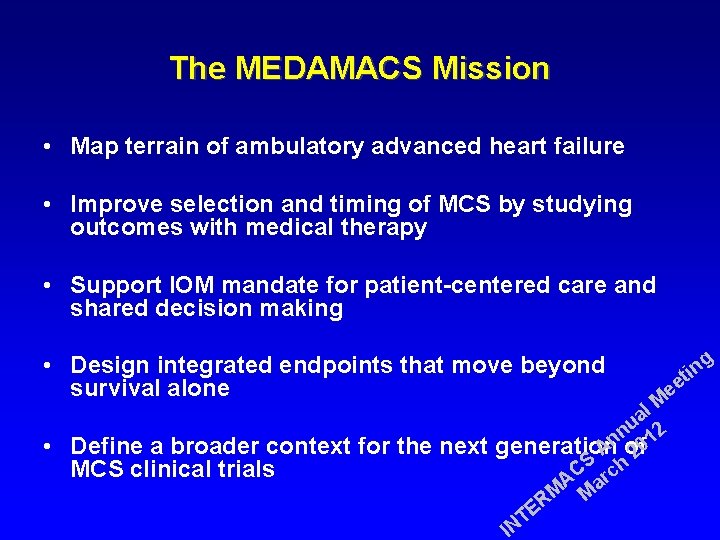
The MEDAMACS Mission • Map terrain of ambulatory advanced heart failure • Improve selection and timing of MCS by studying outcomes with medical therapy • Support IOM mandate for patient-centered care and shared decision making • Design integrated endpoints that move beyond survival alone • g l M a u 2 n n 01 Define a broader context for the next generation of A 2 S h MCS clinical trials C c r A a M M R E T IN in t ee

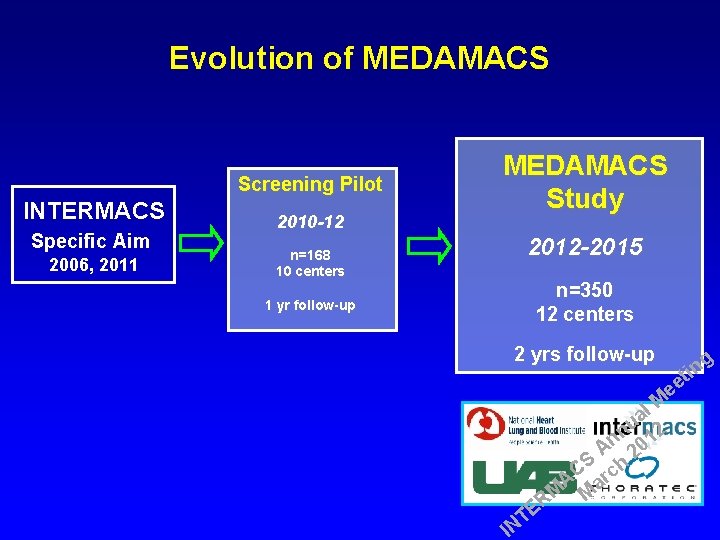
Evolution of MEDAMACS Screening Pilot INTERMACS Specific Aim 2006, 2011 2010 -12 n=168 10 centers 1 yr follow-up MEDAMACS Study 2012 -2015 n=350 12 centers 2 yrs follow-up E T IN g l M a u 2 n n 01 A 2 S h C c r A a M M R in t ee

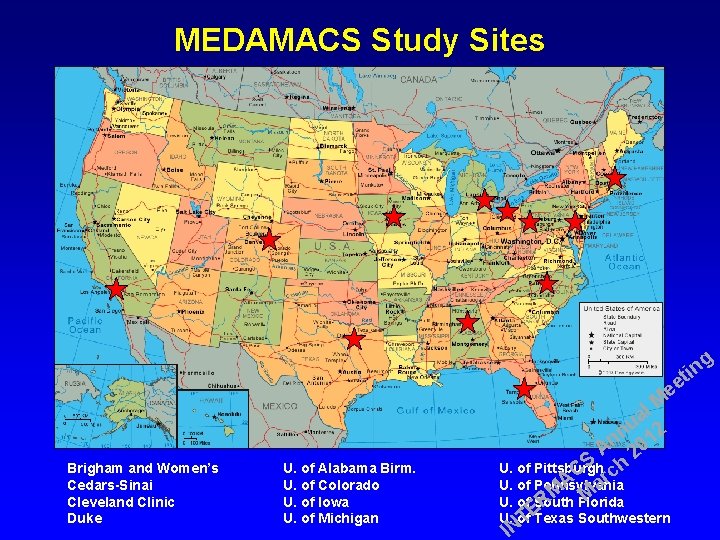
MEDAMACS Study Sites g in t ee Brigham and Women’s Cedars-Sinai Cleveland Clinic Duke U. of Alabama Birm. U. of Colorado U. of Iowa U. of Michigan l M a u 2 n n 01 A 2 S h U. of Pittsburgh C c r A a U. of Pennsylvania M M U. of South Florida R E U. of Texas Southwestern T IN

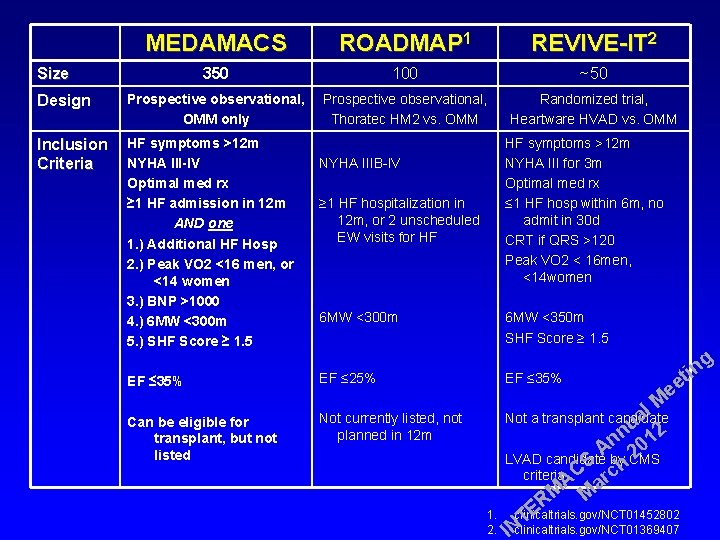
MEDAMACS ROADMAP 1 REVIVE-IT 2 350 100 ~50 Prospective observational, OMM only Prospective observational, Thoratec HM 2 vs. OMM Randomized trial, Heartware HVAD vs. OMM Size Design Inclusion HF symptoms >12 m NYHA III-IV Criteria HF symptoms >12 m NYHA III for 3 m Optimal med rx ≤ 1 HF hosp within 6 m, no admit in 30 d CRT if QRS >120 Peak VO 2 < 16 men, <14 women NYHA IIIB-IV Optimal med rx ≥ 1 HF admission in 12 m ≥ 1 HF hospitalization in 12 m, or 2 unscheduled AND one EW visits for HF 1. ) Additional HF Hosp 2. ) Peak VO 2 <16 men, or <14 women 3. ) BNP >1000 6 MW <300 m 4. ) 6 MW <300 m 5. ) SHF Score ≥ 1. 5 6 MW <350 m SHF Score ≥ 1. 5 EF ≤ 35% EF ≤ 25% EF ≤ 35% Can be eligible for transplant, but not listed Not currently listed, not planned in 12 m a Not a transplant candidate 1. 2. g in t ee l M u 2 n n 01 A by CMS 2 LVAD candidate S h criteria C rc A a M M R E clinicaltrials. gov/NCT 01452802 T INclinicaltrials. gov/NCT 01369407

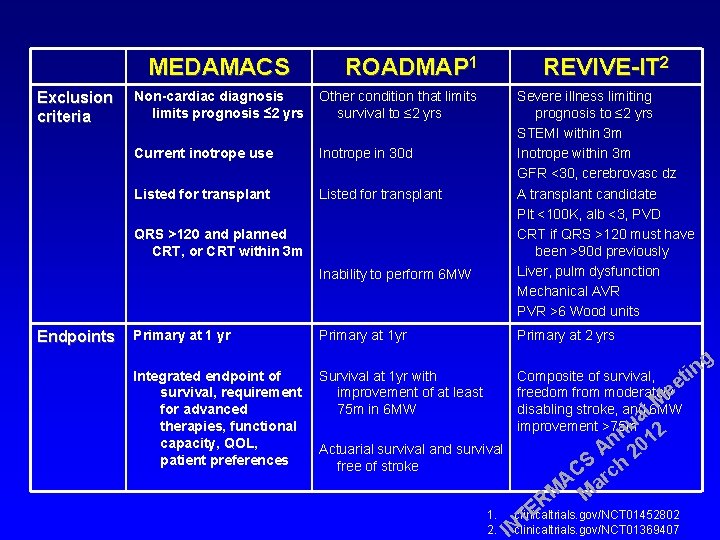
MEDAMACS Exclusion criteria ROADMAP 1 Non-cardiac diagnosis limits prognosis ≤ 2 yrs Other condition that limits survival to ≤ 2 yrs Current inotrope use Inotrope in 30 d Listed for transplant QRS >120 and planned CRT, or CRT within 3 m Inability to perform 6 MW Endpoints Primary at 1 yr Integrated endpoint of survival, requirement for advanced therapies, functional capacity, QOL, patient preferences Primary at 1 yr REVIVE-IT 2 Severe illness limiting prognosis to ≤ 2 yrs STEMI within 3 m Inotrope within 3 m GFR <30, cerebrovasc dz A transplant candidate Plt <100 K, alb <3, PVD CRT if QRS >120 must have been >90 d previously Liver, pulm dysfunction Mechanical AVR PVR >6 Wood units Primary at 2 yrs g in Survival at 1 yr with Composite of survival, t e improvement of at least freedom from moderatelye M 75 m in 6 MW disabling stroke, and l 6 MW a improvement >75 mu n 12 n Actuarial survival and survival A 20 S h free of stroke C A arc M M R E 1. clinicaltrials. gov/NCT 01452802 T 2. IN clinicaltrials. gov/NCT 01369407

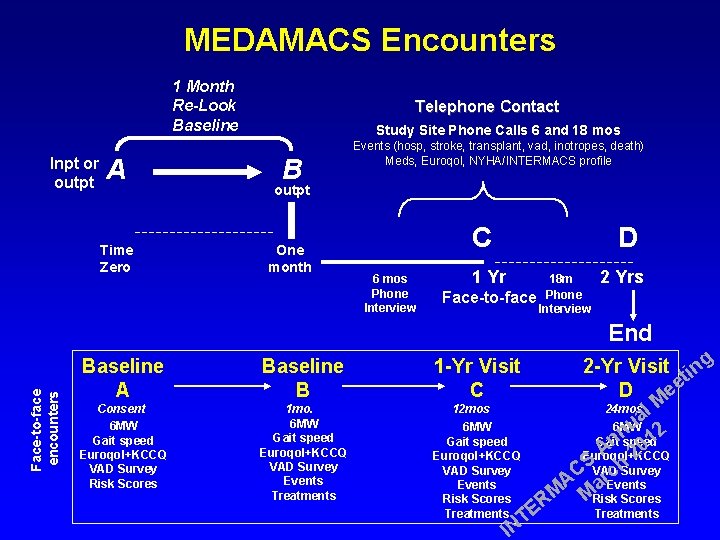
MEDAMACS Encounters 1 Month Re-Look Baseline Inpt or outpt A Time Zero Telephone Contact Study Site Phone Calls 6 and 18 mos B Events (hosp, stroke, transplant, vad, inotropes, death) Meds, Euroqol, NYHA/INTERMACS profile outpt One month C 6 mos Phone Interview D 1 Yr 18 m 2 Yrs Face-to-face Phone Interview Face-to-face encounters End Baseline A Baseline B 1 -Yr Visit C Consent 6 MW Gait speed Euroqol+KCCQ VAD Survey Risk Scores 1 mo. 6 MW Gait speed Euroqol+KCCQ VAD Survey Events Treatments 12 mos 6 MW Gait speed Euroqol+KCCQ VAD Survey Events Risk Scores Treatments E T IN 2 -Yr Visit ting D ee 24 mos l M a u 6 MW n 12 Gait speed n A 20 Euroqol+KCCQ SVAD Survey h C c r A a Events M MRisk Scores R Treatments

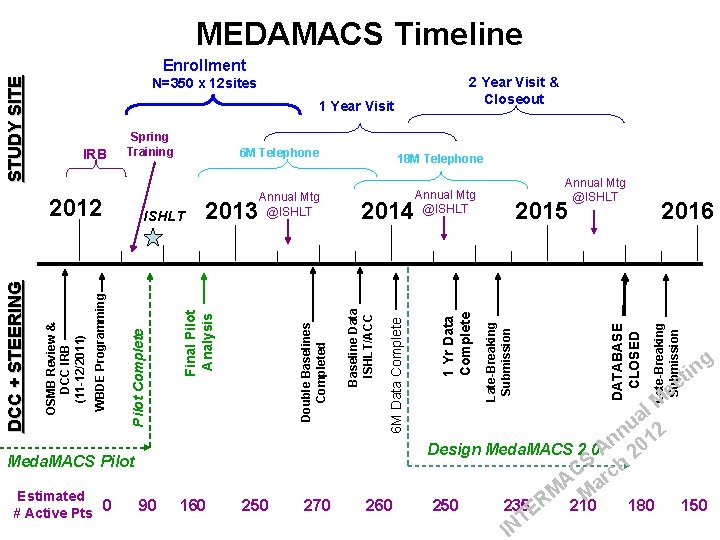
Enrollment 1 Year Visit Meda. MACS Pilot Estimated 0 90 # Active Pts DATABASE CLOSED Late-Breaking Submission 2015 2016 Late-Breaking Submission Annual Mtg @ISHLT 1 Yr Data Complete 2014 6 M Data Complete 2013 Annual Mtg @ISHLT 18 M Telephone Baseline Data ISHLT/ACC ISHLT Pilot Complete WBDE Programming OSMB Review & DCC IRB (11 -12/2011) 6 M Telephone Double Baselines Completed IRB Spring Training 2012 DCC + STEERING 2 Year Visit & Closeout N=350 x 12 sites Final Pilot Analysis STUDY SITE MEDAMACS Timeline g in t ee l M a u 2 n 1 Design Meda. MACS 2. 0 An 20 S h AC arc M M R 160 250 270 260 250 235 210 180 150 E T IN

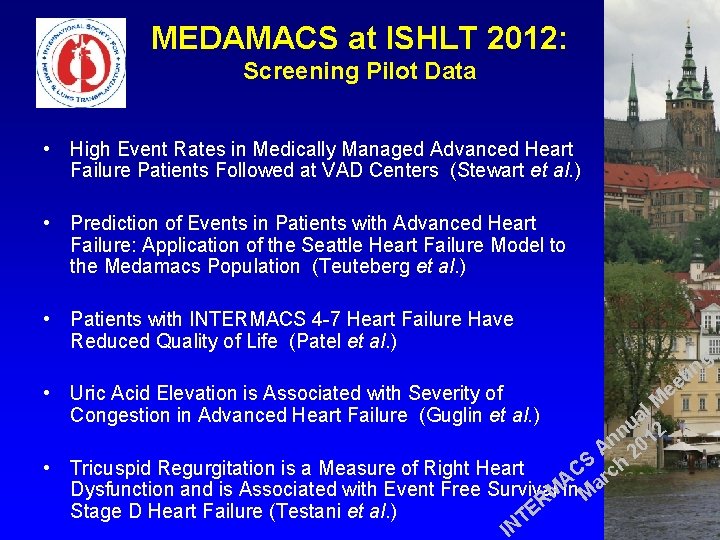
MEDAMACS at ISHLT 2012: Screening Pilot Data • High Event Rates in Medically Managed Advanced Heart Failure Patients Followed at VAD Centers (Stewart et al. ) • Prediction of Events in Patients with Advanced Heart Failure: Application of the Seattle Heart Failure Model to the Medamacs Population (Teuteberg et al. ) • Patients with INTERMACS 4 -7 Heart Failure Have Reduced Quality of Life (Patel et al. ) • Uric Acid Elevation is Associated with Severity of Congestion in Advanced Heart Failure (Guglin et al. ) g l M a u 2 n n 01 A 2 S h • Tricuspid Regurgitation is a Measure of Right Heart C c r A a Dysfunction and is Associated with Event Free Survival. Min M R E Stage D Heart Failure (Testani et al. ) T IN in t ee

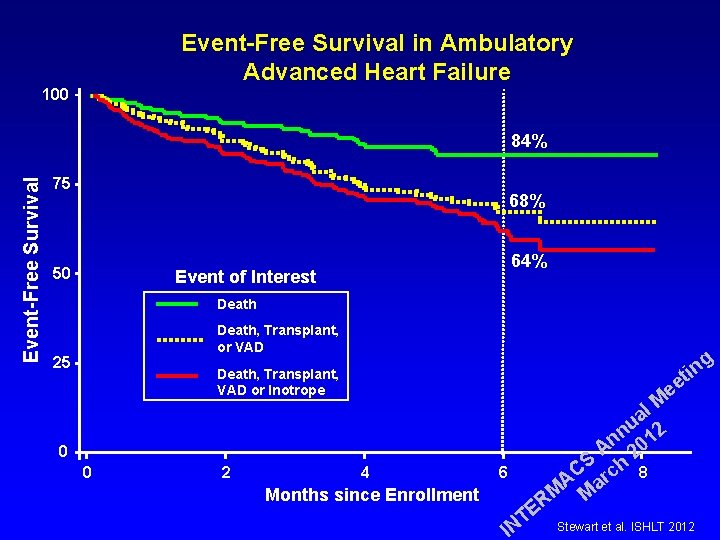
Event-Free Survival in Ambulatory Advanced Heart Failure 100 Event-Free Survival 84% 75 68% 50 64% Event of Interest Death, Transplant, or VAD 25 g in t ee Death, Transplant, VAD or Inotrope 0 0 2 4 Months since Enrollment 6 E T IN l M a u 2 n n 01 A 2 S h 8 C c r A a M M R Stewart et al. ISHLT 2012

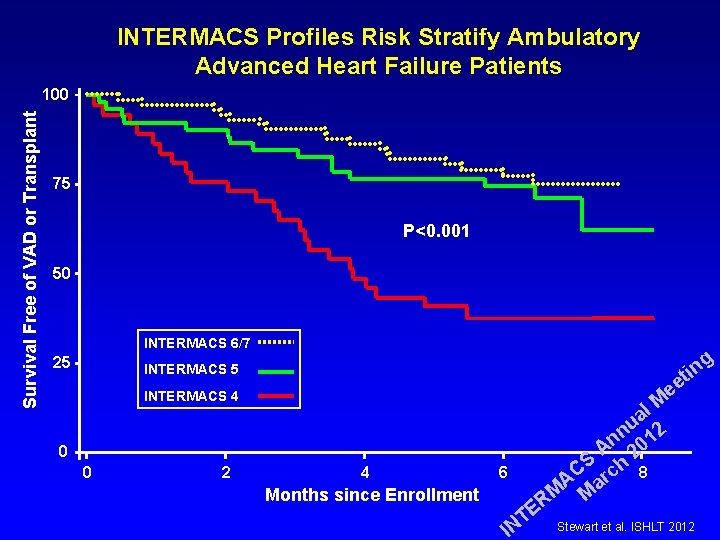
INTERMACS Profiles Risk Stratify Ambulatory Advanced Heart Failure Patients Survival Free of VAD or Transplant 100 75 P<0. 001 50 INTERMACS 6/7 25 g in t ee INTERMACS 5 INTERMACS 4 0 0 2 4 Months since Enrollment 6 E T IN l M a u 2 n n 01 A 2 S h 8 C c r A a M M R Stewart et al. ISHLT 2012

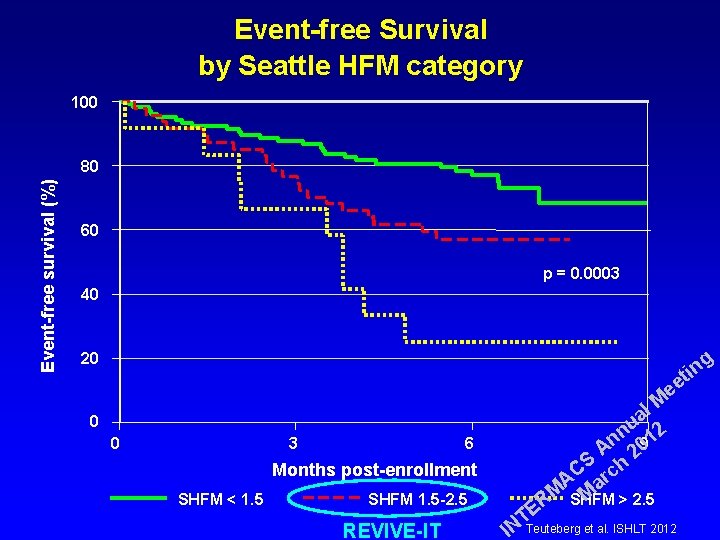
Event-free Survival by Seattle HFM category 100 Event-free survival (%) 80 60 p = 0. 0003 40 g 20 in t ee 0 0 3 6 Months post-enrollment SHFM < 1. 5 SHFM 1. 5 -2. 5 REVIVE-IT l M a u 2 n n 901 A 2 S h C c r A a M M R SHFM > 2. 5 E T IN Teuteberg et al. ISHLT 2012

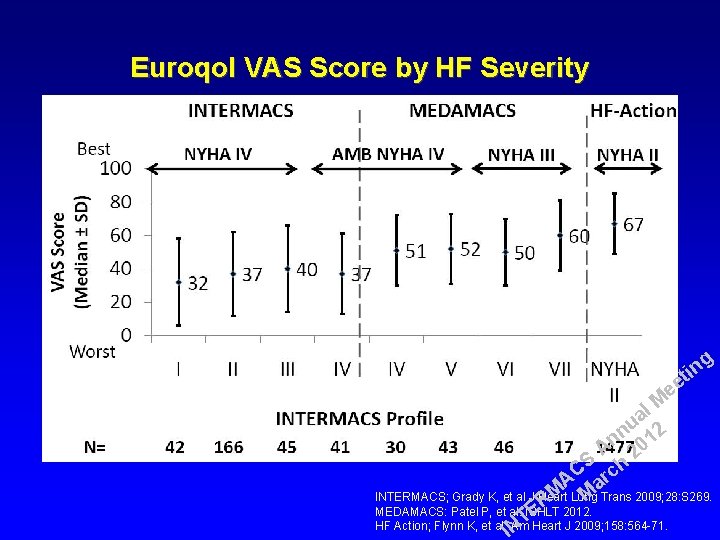
Euroqol VAS Score by HF Severity g in t ee l M a u 2 n n 01 A 2 S h C c r A a M INTERMACS; Grady K, et al J Heart Lung M Trans 2009; 28: S 269. R MEDAMACS: Patel P, et al. ISHLT 2012. E T HF Action; Flynn K, et al. Am IN Heart J 2009; 158: 564 -71.

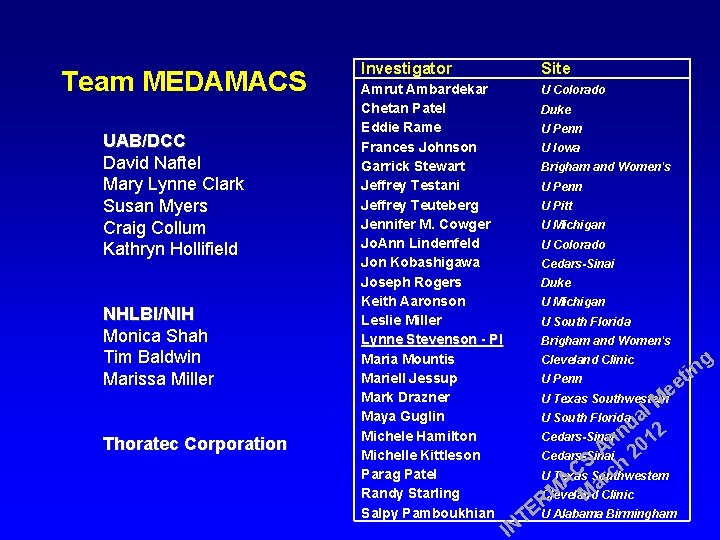
Team MEDAMACS UAB/DCC David Naftel Mary Lynne Clark Susan Myers Craig Collum Kathryn Hollifield NHLBI/NIH Monica Shah Tim Baldwin Marissa Miller Thoratec Corporation Investigator Site Amrut Ambardekar Chetan Patel Eddie Rame Frances Johnson Garrick Stewart Jeffrey Testani Jeffrey Teuteberg Jennifer M. Cowger Jo. Ann Lindenfeld Jon Kobashigawa Joseph Rogers Keith Aaronson Leslie Miller Lynne Stevenson - PI Maria Mountis Mariell Jessup Mark Drazner Maya Guglin Michele Hamilton Michelle Kittleson Parag Patel Randy Starling Salpy Pamboukhian U Colorado Duke U Penn U Iowa Brigham and Women's U Penn U Pitt U Michigan U Colorado Cedars-Sinai Duke U Michigan U South Florida Brigham and Women's Cleveland Clinic U Penn l M a u 2 Cedars-Sinainn 1 0 A 2 Cedars-Sinai S h C c U Texas Southwestern r A a M Cleveland M Clinic R EU Alabama Birmingham T IN U Texas Southwestern U South Florida g in t ee

Coordinator Training Session: March 11, 2012 pedi. MACS David C. Naftel, Ph. D g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R 39

Coordinator Training Session: March 11, 2012 INTERMACS Re-Launch Status § Our Goals: § § Reduce the number of elements and forms Streamline the data entry process Clarify elements that were confusing in the past Examine the AE definitions for current clinical relevance g it n • It became clear that the pediatric VAD patients needed ee a different web-based data entry system. al M E T IN u 2 n n 01 A 2 S h C c r A a M M R 40

Coordinator Training Session: March 11, 2012 pedi. MACS Launch Status § Pediatric Committee § § § Betsy Blume, MD - Chair David Morales, MD David Rosenthal, MD Peter Wearden, MD Christopher Almond, MD Robert Jaquiss, MD Jonathan Chen, MD Dee Epstein, RN Heidi Moses, MEd, CCRA David Naftel, Ph. D Tim Baldwin, Ph. D g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R 41

Coordinator Training Session: March 11, 2012 pedi. MACS Launch Status § We (Pediatric Committee, NIH, INTERMACS Co-PIs) have spent 1 year reviewing the WBDE in all aspects: § § AE definitions and other definitions Screens / Forms Data Elements Patient Flow through the WBDE g in t ee E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R 42

Coordinator Training Session: March 11, 2012 pedi. MACS Launch Status § pedi. MACS will follow the structure of INTERMACS § A few important changes from INTERMACS: § Pediatric patients (< 19 yrs. at time of implant) § Includes both durable and temporary support MCSDs g it n § Modifications of AE definitions ee l M a § Possible expansion of quality of life instrumentsnu 2 1 E T IN n 0 A 2 S h C c r A a M M R 43

Coordinator Training Session: March 11, 2012 pedi. MACS Launch Status Target: Live Test Site July 1, 2012 Jul 1 - Jul 31 Target: LAUNCH Date Aug 1, 2012 § Target Live test site: July 1, 2012 § § § Testing by INTERMACS Nurse Monitors Testing by the DCC Data Managers Testing by the INTERMACS Co-PIs Testing by 3 Hospitals (Beta Sites) Testing by Pediatric Committee g in t ee § Target Launch Date: August 1, 2012 § Online training will be available § Training Session in September 2012 E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R 44

Coordinator Training Session: March 11, 2012 pedi. MACS Launch Status § Training Session for pedi. MACS § “Mechanical Cardiac Support in Pediatric Heart Disease – State of the Art 2012”: September 20 -22, 2012 § The St. Louis Children’s and Washington University g in t ee Heart Center E T IN l M a u 2 n n 01 A 2 S h C c r A a M M R 45
 Peter piper tongue twister
Peter piper tongue twister The sixth sick sheik's sixth sheep's sick lyrics
The sixth sick sheik's sixth sheep's sick lyrics Anthem of poland
Anthem of poland March 7, 2012
March 7, 2012 Informs annual meeting
Informs annual meeting Aupha annual meeting
Aupha annual meeting American psychiatric association annual meeting 2020
American psychiatric association annual meeting 2020 Ky masonic lodges
Ky masonic lodges Positron vs proton
Positron vs proton Cwemf
Cwemf How to run an annual general meeting
How to run an annual general meeting Nrg oncology meeting 2017
Nrg oncology meeting 2017 Aashto annual meeting 2015
Aashto annual meeting 2015 Scts membership
Scts membership Nrg oncology semiannual meeting
Nrg oncology semiannual meeting American epilepsy society annual meeting 2017
American epilepsy society annual meeting 2017 For today's meeting
For today's meeting Proposal kickoff meeting agenda
Proposal kickoff meeting agenda What is meeting and types of meeting
What is meeting and types of meeting What is meeting and types of meeting
What is meeting and types of meeting Shaala siddhi annual attendance rate
Shaala siddhi annual attendance rate Key partners bmc example
Key partners bmc example Business model canvas tripadvisor
Business model canvas tripadvisor Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ