Semiannual Meeting Plenary Session January 9 11 2020

































- Slides: 39

Semiannual Meeting Plenary Session January 9 -11, 2020 Marriott Marquis Houston NRG Oncology @NRGOnc #NRG 2020

NRG Oncology Plenary Session 10 am – 12 pm @NRGonc #NRG 2020

Advancing Research. Improving Lives. NRG Oncology seeks to improve the lives of cancer patients through its practice-changing multiinstitutional clinical and translational research. @NRGOnc #NRG 2020

NRG Oncology 2019 In Review @NRGOnc #NRG 2020

NRG Oncology 2019 Summary • • Year 1 (of 6) of 1 st Competitive Renewal (Score 16) Highest Ever Number of NRG Trials Activated Strong Enrollment across all Committees & Sites Number of High Level Publicatons (NEJM, Lancet) Success with NCTN Navigator System NRG NCORP Grant Renewed NRG Biospecimen Bank Grant Submitted @NRGOnc #NRG 2020

NRG Oncology Membership • 124 Main Members • 32 LAPS • 29 NCORP Sites • 276 Affiliate Members 1868 Participating Sites @NRGOnc #NRG 2020

NRG Oncology 2019 Accrual • 2254 patients accrued to NRG-led trials • 2820 patient accruals credited to NRG by NRG members for studies led by other groups @NRGOnc #NRG 2020

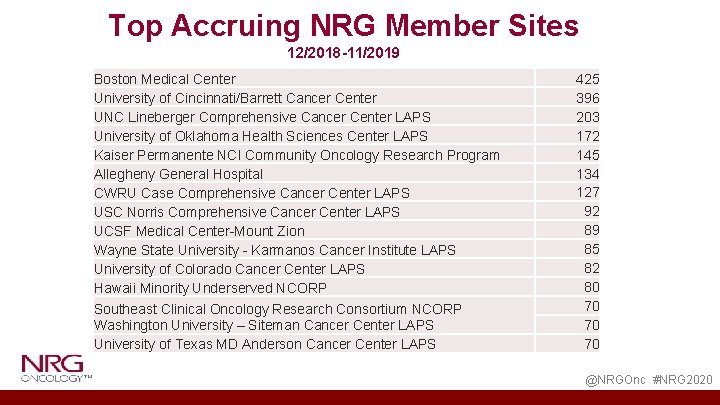
Top Accruing NRG Member Sites 12/2018 -11/2019 Boston Medical Center University of Cincinnati/Barrett Cancer Center UNC Lineberger Comprehensive Cancer Center LAPS University of Oklahoma Health Sciences Center LAPS Kaiser Permanente NCI Community Oncology Research Program Allegheny General Hospital CWRU Case Comprehensive Cancer Center LAPS USC Norris Comprehensive Cancer Center LAPS UCSF Medical Center-Mount Zion Wayne State University - Karmanos Cancer Institute LAPS University of Colorado Cancer Center LAPS Hawaii Minority Underserved NCORP Southeast Clinical Oncology Research Consortium NCORP Washington University – Siteman Cancer Center LAPS University of Texas MD Anderson Cancer Center LAPS 425 396 203 172 145 134 127 92 89 85 82 80 70 70 70 @NRGOnc #NRG 2020

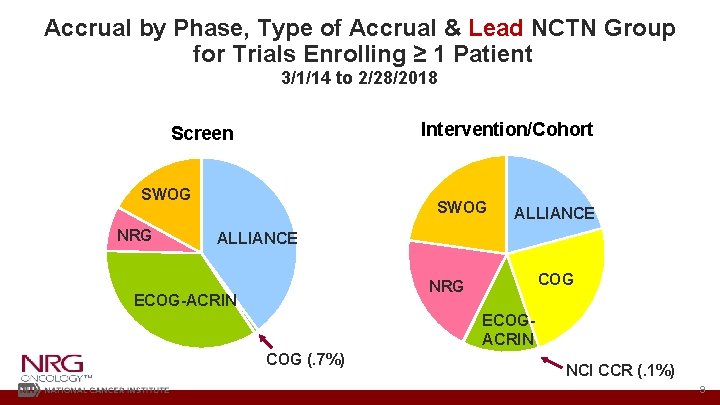
Accrual by Phase, Type of Accrual & Lead NCTN Group for Trials Enrolling ≥ 1 Patient 3/1/14 to 2/28/2018 Intervention/Cohort Screen SWOG NRG SWOG ALLIANCE COG NRG ECOG-ACRIN ECOGACRIN COG (. 7%) NCI CCR (. 1%) 9

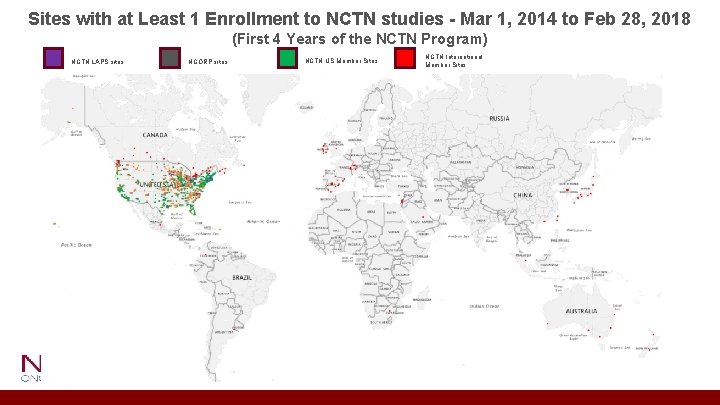
Sites with at Least 1 Enrollment to NCTN studies - Mar 1, 2014 to Feb 28, 2018 (First 4 Years of the NCTN Program) NCTN LAPS sites NCORP sites NCTN US Member Sites NCTN International Member Sites

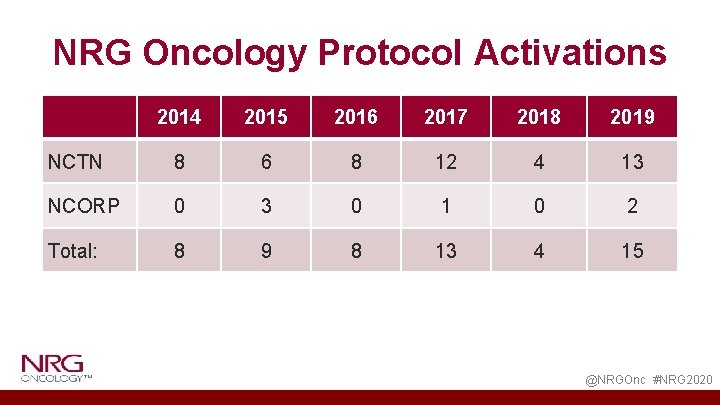
NRG Oncology Protocol Activations 2014 2015 2016 2017 2018 2019 NCTN 8 6 8 12 4 13 NCORP 0 3 0 1 0 2 Total: 8 9 8 13 4 15 @NRGOnc #NRG 2020

2019 NRG Trial Activations Study # Title NRG-LU 004 Ph I accelerated or conventional RT +durvalumab for patients with PD-L 1 High Locally Advanced NSCLC (ARCHON-1) NRG-GI 006 Ph III Randomized Chemo-Proton Vs Chemo-IMRT for Esophageal Cancer NRG-BR 004 Rand, Ph III of Paclitaxel/Trastuzumab/Pertuzumab with Atezolizumab or Placebo for women with First-Line HER 2+ Metastatic Breast Cancer – Registration-Intent trial NRG-CC 007 -CD Increasing the Dose of Survivorship Care Planning for Prostate Cancer Survivors Who Receive Androgen Deprivation Therapy – NCORP Cancer Care Delivery Research Trial NRG-GY 014 Ph II Tazemetostat (EPZ-6438) for Women with Recurrent or Persistent Endometrioid or Clear Cell Carcinoma of the Ovary or Recurrent or Persistent Endometrioid Endometrial Adenocarcinoma NRG-LU 003 A Biomarker-Driven Protocol for Previously Treated ALK-Positive Non. Squamous NSCLC Patients @NRGOnc #NRG 2020

2019 NRG Trial Activations (Cont) Study # Title NRG-LU 005 Limited Stage Small Cell Lung Cancer Patients: Ph II/III Chemo-RT with vs without Atezolizumab – Registration-Intent trial NRG-CC 006 Ancillary Study to BR 005 - Limited to University of Michigan – NCORP study NRG-GU 007 Ph IIR Niraparib w RT and ADT for Men with High Risk Prostate Cancer (With Initial Phase I) NRG-HN 005 Ph II/III De-intensified RT for Early-Stage, p 16+, Non-Smoking Associated Oropharyngeal Cancer Patients NRG-GY 018 Ph. IIIR, Placebo-Controlled Pembrolizumab in Addition to Paclitaxel+Carboplatin for Women with Stage III or IVA, Stage IVB or Recurrent Endometrial Cancer – Registration-Intent trial NRG-GY 019 RPh III, 2 -Arm Trial of Paclitaxel/Carboplatin/Maintenance Letrozole Vs Letrozole Monotherapy for Women with Stage II-IV, Primary Low-Grade Serous Carcinoma of the Ovary or Peritoneum @NRGOnc #NRG 2020

2019 NRG Trial Activations (Cont) Study # Title NRG-GY 021 A Phase II Randomized Trial of Olaparib Vs Olaparib Plus Tremelimumab for Women with Platinum-Sensitive Recurrent Ovarian Cancer NRG-GY 022 Assessment of Carboplatin Clearance Predictors: A PK Study on NCISponsored Clinical Trials or Standard of Care Treatments Using Carboplatin NRG-GI 005 Phase II/III Study of Circulating Tum. Or DNA as a Predictive Bioma. Rker in Adjuvant Chemotherapy for Patients with Stage IIA Colon Cancer (COBRA) SWOG/NRG S 1806 Phase III Randomized Trial of Concurrent Chemo-RT with vs without Atezolizumab for Pts with Localized Muscle Invasive Bladder Cancer SWOG/NRG Joint study: SWOG is lead) - Registration-Intent trial Total 16 studies (13 CTEP, 2 NCORP, 1 joint study w/SWOG) 15 NRG-led trials w/ 1 st CCDR study and 3 registration-intent studies @NRGOnc #NRG 2020

• NRG Oncology has submitted 21 projects through the NCTN Navigator system since 3/1/2019 @NRGOnc #NRG 2020

NRG-RTOG 1216 - Randomized Phase II/III Trial of Surgery and Postoperative Radiation Delivered with Concurrent Cisplatin Versus Docetaxel Versus Cetuximab and Docetaxel for High-Risk Squamous Cell Cancer of the Head and Neck BIQSFP NRG-BN 001 - MGMT Promoter Hyperthylation by Standard q. MSPCR as Integral Biomarker NRG-BN 003 - p. HH 3 Mitotic Index as an Integrated Marker for patients treated on NRG-BN 003, "Phase III Trial of Observation vs Irradiation for a Gross Totally Resected Grade II Meningioma". NRG-HN 001 - Integral Determination of Pre- and Post-treatment EBV DNA in EBV+ Nasopharyngeal Ca for Risk Group Stratification NRG-LU 003 – 1) Next Generation Sequencing for Patient Assignment to Treatment Cohort; 2) Correlation of Genetic Alterations in cf. DNA versus Tumor Tissue for Next Generation Sequencing for Patient Assignment to Treatment Cohort @NRGOnc #NRG 2020

NRG-HN 1903 - Determining Negative Predictive Value of FDG PET/CT for N 0 Neck for T 1 -T 2 Oral cavity Sq. Cell. Ca Patients (BIQSFP PI: Rathan M. Subramaniam, MD, Ph. D) BIQSFP New Awards in 2019 NRG-BN 1855 - A Randomized Phase II/III Open Label Study of Ipilimumab and Nivolumab vs Temozolomide for Patients with Newly Unmethylated MGMT (Tumor O-6 -methylguanine DNA Methyltransferase) Glioblastoma (BIQSFP PI: Eric Sulman, MD, PHD) NRG-GY 012 - Integrated Biomarker Studies Utilizing Homologous Recombination Deficiency (HRD) and Plasma Angiome for Women with Recurrent, Persistent or Metastatic Endometrial Cancer Enrolled on NRG-GY 012 (BIQSFP PI: Douglas Levine, M. D. ) Biomarkers for Prediction of Radiation-Induced Neurocognitive Toxicity (BIQSFP PI: Vinai Gondi, M. D. ) @NRGOnc #NRG 2020

NRG Oncology Biospeciman Bank Grant • 6 Year Renewal Application Submitted December 17, 2019 @NRGOnc #NRG 2020

NRG Oncology Biospeciman Bank Grant • NRG Oncology Biospecimen Bank supports NRG Oncology’s research, including translational research by providing the expertise, experience, and infrastructure needed for the collection, storage, and distribution of high quality biospecimens for all types of clinical trials and translational (correlative science) research. • Since 2015, 254, 639 biospecimens have been collected from 14, 356 patients and 86, 053 biospecimens have been distributed to 194 investigators for integral or integrated translational science. This work has led to 83 publications. @NRGOnc #NRG 2020

N R G Richard C. K. Jordan, DDS, Ph. D B i o b Nilsa Ramirez-Milan, MD Peter C. Lucas, MD, Ph. D @NRGOnc #NRG 2020

NRG Oncology Leadership Transitions • Gastrointestinal Cancer Committee • Developmental Therapeutics Committee • Radiation Oncology Subcommittee • Protocol Support Committee @NRGOnc #NRG 2020

NRG Oncology Gastrointestinal Cancer Committee Christopher Crane, MD Memorial Sloan Kettering Cancer Center Theodore S. Hong, MD Massachusetts General Hospital @NRGOnc #NRG 2020

NRG Oncology Developmental Therapeutics – RT Subcommittee David Raben, MD U Colorado Steven Lin, M. D. , Ph. D UT MD Anderson Cancer Center Sandip Patel, MD UC San Diego Health @NRGOnc #NRG 2020

N R G O n c o l o Thank You Susan Nolte CRNP, Ph. D Chair PSC Nancy Knudsen, RN Co-Chair, PSC @NRGOnc #NRG 2020

N R G O n c o l o Welcome Terry Thomas, MS, CCRC Chair, PSC Nancy Fusco, RN, BSN, CCRP Vice-Chair, PSC Cynthia Licavoli, RN, BSN, MA Vice-Chair, PSC @NRGOnc #NRG 2020

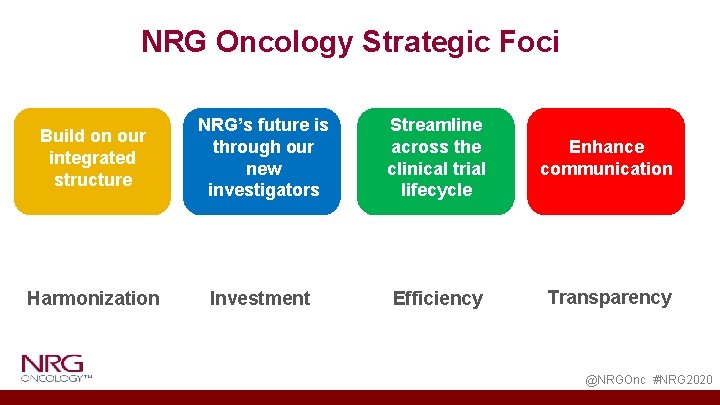
NRG Oncology Strategic Foci Build on our integrated structure NRG’s future is through our new investigators Streamline across the clinical trial lifecycle Enhance communication Harmonization Investment Efficiency Transparency @NRGOnc #NRG 2020

Meeti n g s t T a e w r t e e R a e k e t t s i m m o C s d r a w A l e v a r T @NRGOnc #NRG 2020

@NRGOnc #NRG 2020

NRG Oncology Machtay Travel Award - Endowed Gift - Travel support for one new investigator to attend an NRG Oncology meeting each year Mitchell Machtay, MD Deputy Group Chair, NRG Oncology Chair, Radiation Oncology, Case Western Reserve University @NRGOnc #NRG 2020

Support NRG’s Mission! You can donate RIGHT NOW Text NRG 2020 to 41444 Complete the form, including amount you wish to donate @NRGOnc #NRG 2020

Outstanding NRG Publications 2019 @NRGOnc #NRG 2020

Outstanding NRG Publication 2019 NRG-GOG 0244 – Jeanne Carter, et al. Gynecol Oncol 12/2019 GOG 244 - The Lymph. Edema and Gynecologic Cancer (LEG) Study: The Association between the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) and Lymphedema of the Lower Extremity (LLE). NRG-GOG 9929 - Jyoti Mayadev, et al. JAMA Oncol 11/27/2019 Sequential Ipilimumab After Chemoradiotherapy in Curative-Intent Treatment of Patients With Node. Positive Cervical Cancer. NRG-GOG-0199 - Pl Mai, et al. Gynecol Oncol 11/21/2019 Prospective Follow-up of Quality of Life for Participants Undergoing Risk-reducing Salpingo-oophorectomy Orovarian Cancer Screening in GOG-0199. NRG-GOG-0213 - Robert Coleman, et al. N Engl J Med 11/14/2019 Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. @NRGOnc #NRG 2020

Outstanding NRG Publications 2019 NRG-GOG-0258 - Daniela Matei, et al. N Engl J Med 6/13/2019 Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. NRG-GOG-0281 - David Gershenson, et al. ESMO 9/27/2019 A Randomized Phase II/III Study to Assess the Efficacy of Trametinib in Patients with Recurrent or Progressive Low-Grade Serous Ovarian Cancer or Peritoneal Cancer. NRG-HN 002 - Sue Yom, et al. ASTRO September 2019 NRG-HN 002: A Randomized Phase II trial for Patients with P 16 Positive, Non-smoking Associated, Locoregionally Advanced Oropharyngeal Cancer. NRG-NSABP B-39 – Frank Vicini, et al. Lancet 12/14/2019 Long-term Primary Results of Accelerated Partial Breast Irradiation after Breast-conserving Surgery for Early-stage Breast Cancer: A Randomised, Phase 3, Equivalence Trial. @NRGOnc #NRG 2020

Outstanding NRG Publications 2019 NRG-RTOG 0438 - Laura Dawson, et. al. Pract Radiat Oncol Jul - Aug 2019 NRG Oncology/RTOG 0438: A Phase 1 Trial of Highly Conformal Radiation Therapy for Liver Metastases. NRG-RTOG 0617 - Jeffrey Bradley, et al. J Clin Oncol 12/16/2019 Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. NRG-RTOG 9811 - David Konieczkowski, et al. ASTRO September 2019 The Genomic Landscape of Anal Cancer and its Relationship to Clinical Outcomes: An Exploratory Analysis of NRG Oncology/RTOG 98 -11. Nie K, et al. Int J Radiat Oncol Biol Phys 6/1/2019 NCTN Assessment on Current Applications of Radiomics in Oncology. @NRGOnc #NRG 2020

NRG Oncology 2019 Summary • • Year 1 (of 6) of 1 st Competitive Renewal (Score 16) Highest Ever Number of NRG Trials Activated Strong Enrollment across all Committees & Sites Number of High Level Publicatons (NEJM, Lancet) Success with NCTN Navigator System NRG NCORP Grant Renewed NRG Biospecimen Bank Grant Submitted @NRGOnc #NRG 2020

NRG Oncology 2020 Opportunities • Further Execution of NRG Process Improvement • Harmonization • Investment • Transparency • Efficiency • Sustained High Level of NRG Trial Activations @NRGOnc #NRG 2020

NRG Oncology 2020 Opportunities • • Committee Retreats and Inter-Committee Retreats Expansion of International Collaboration Expanded New Investigator Support New Opportunities with Industry Partners @NRGOnc #NRG 2020

NRG Oncology NCORP Research Base @NRGOnc #NRG 2020

Scientific Session @NRGOnc #NRG 2020 NRG Oncology
 Nrg oncology meeting 2017
Nrg oncology meeting 2017 Nrg oncology semiannual meeting 2021
Nrg oncology semiannual meeting 2021 Nrg oncology meeting 2018
Nrg oncology meeting 2018 Welcome to new session 2020-21
Welcome to new session 2020-21 Preservation in the bible
Preservation in the bible Trigonometry starter
Trigonometry starter Plenary ideas maths
Plenary ideas maths Plenary genitive
Plenary genitive Plenary questions
Plenary questions Plenary power
Plenary power Proposal kickoff meeting agenda
Proposal kickoff meeting agenda What is meeting and types of meeting
What is meeting and types of meeting What is meeting and types of meeting
What is meeting and types of meeting For todays meeting
For todays meeting Rhic ags users meeting 2020
Rhic ags users meeting 2020 Hom herbalife opportunity meeting
Hom herbalife opportunity meeting American psychiatric association annual meeting 2020
American psychiatric association annual meeting 2020 1st january 2018
1st january 2018 1995 january 23 nasa
1995 january 23 nasa Nysedregents
Nysedregents 9 months before january 26 2009
9 months before january 26 2009 Pro forma journal entries example
Pro forma journal entries example January 10th 1776
January 10th 1776 Jill kally
Jill kally 25 january 1759
25 january 1759 Mozart grave
Mozart grave Respect character trait
Respect character trait January february march april may
January february march april may Arvod cannot find work as a mall santa in january.
Arvod cannot find work as a mall santa in january. Sunday, tuesday, january, saturday
Sunday, tuesday, january, saturday December november october
December november october Divide elephant into syllables
Divide elephant into syllables January 24th 1848
January 24th 1848 January 2012 chemistry regents answers
January 2012 chemistry regents answers Mozart nationality
Mozart nationality January 2012
January 2012 Spatial january
Spatial january An asset was purchased for $120 000 on january 1
An asset was purchased for $120 000 on january 1 January february spelling
January february spelling January 2006 calendar
January 2006 calendar