REVISION ELECTROCHEMISTRY OXIDATION Oxidation is a loss of


















- Slides: 28

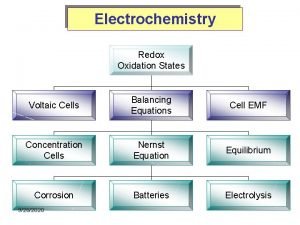
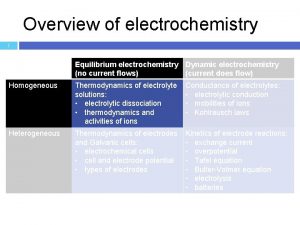
REVISION ELECTROCHEMISTRY

OXIDATION • Oxidation is a loss of electrons • The oxidation number increases • E. g. • Electrodes where oxidation take place will reduce in mass • We write the oxidation reaction ‘upside-down’ from the table • Reducing agent ANODE The electrode where oxidation takes place

REDUCTION • Reduction is a gain of electrons • The oxidation number decreases • E. g. • Electrodes where reduction take place will increase in mass • We write the reduction reaction ‘right-way-round’ from the table • Oxidising agent CATHODE The electrode where reduction takes place

ELECTROLYTE solution/liquid/dissolved substance that conducts electricity through the movement of ions.

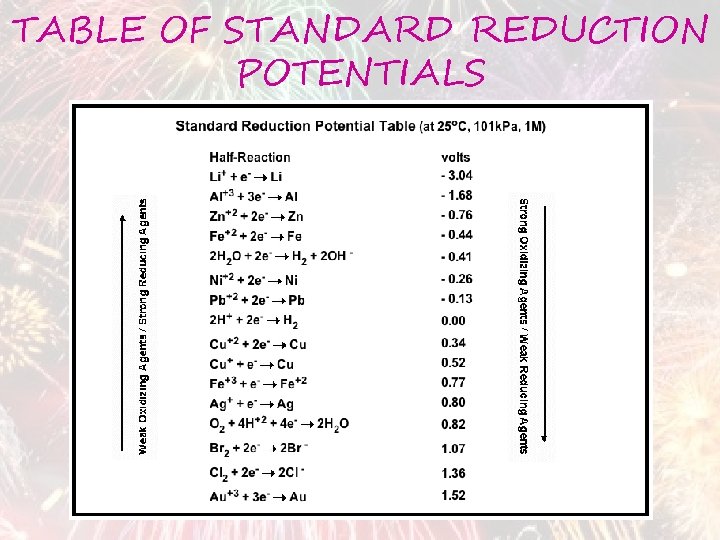
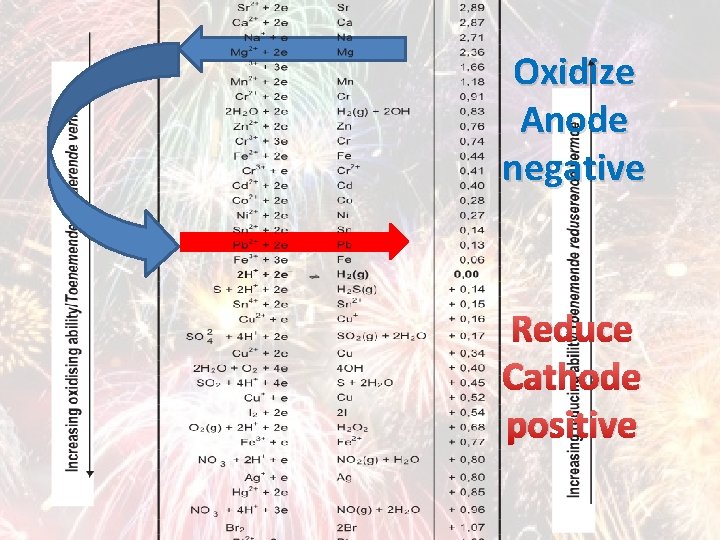
TABLE OF STANDARD REDUCTION POTENTIALS

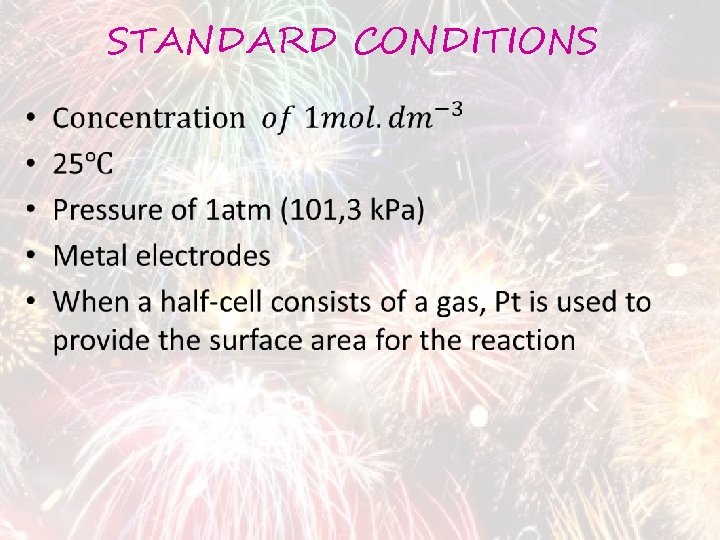
STANDARD CONDITIONS •

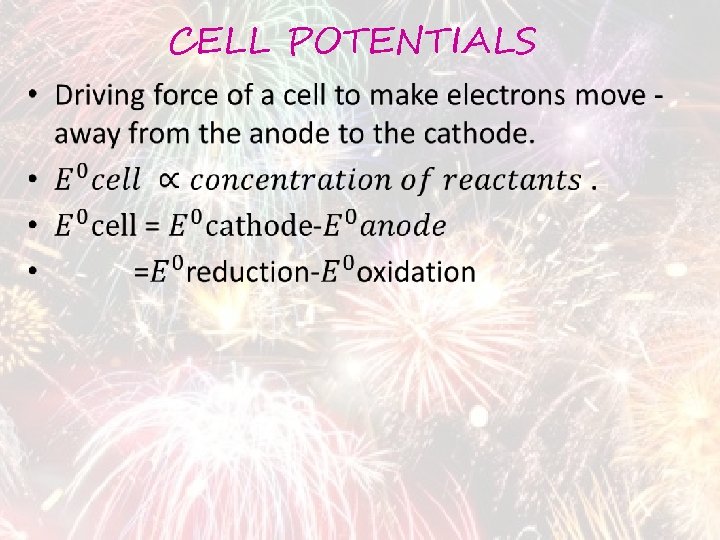
CELL POTENTIALS •

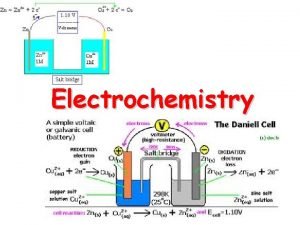
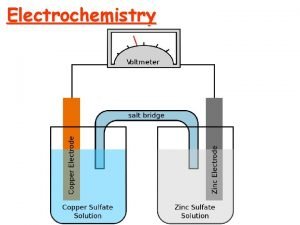
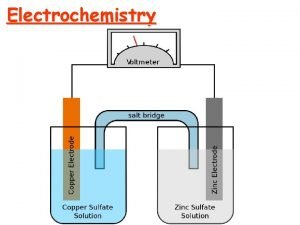
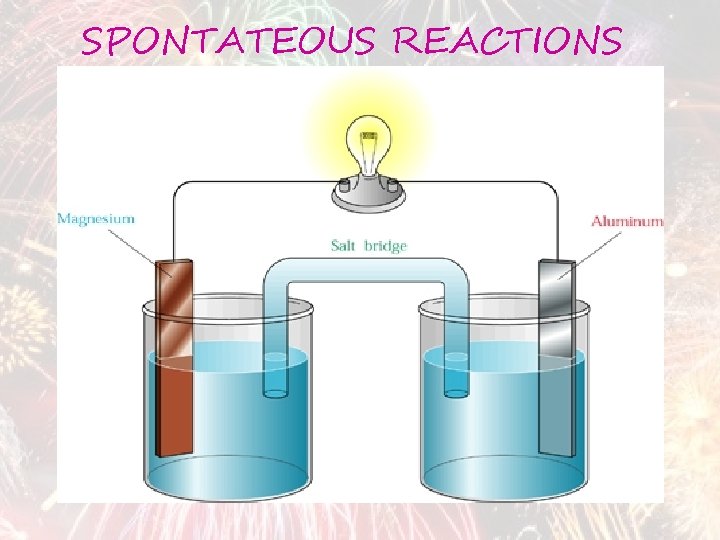
GALVANIC CELL a cell in which chemical energy is converted into electrical energy. A galvanic (voltaic) cell has self-sustaining electrode reactions

SPONTATEOUS REACTIONS

SALT BRIDGE Its function is to maintain ELECTRICAL neutrality

CELL NOTATION • The H 2|H+ half-cell is treated just like any other half-cell. . • Cell terminals (electrodes) are written on the outside of the cell notation. • Active electrodes reducing agent | oxidised species || oxidising agent | reduced species • Inert electrodes (usually Pt or C): Pt | reducing agent | oxidised species || oxidising agent | reduced species | Pt Example: Pt | Cℓ-(aq) |Cℓ 2(g) || F 2(g) | F-(aq) | Pt






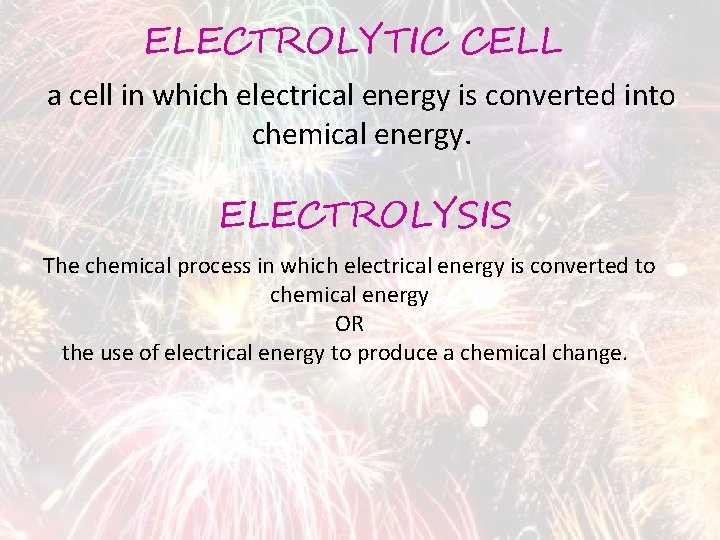
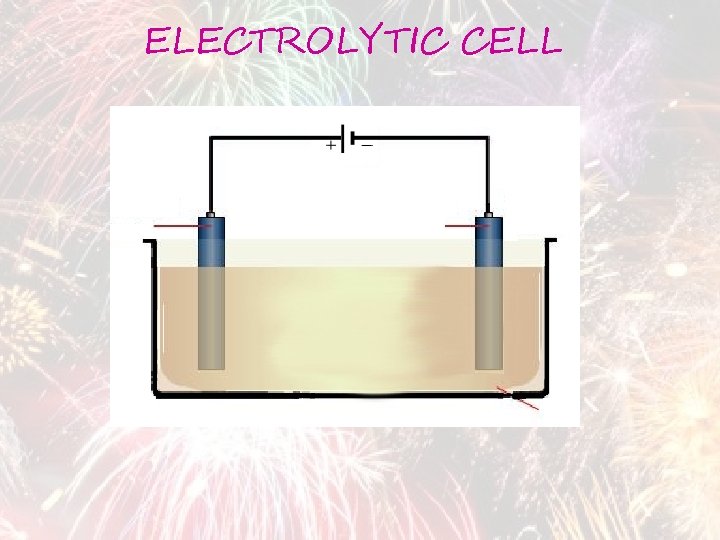
ELECTROLYTIC CELL a cell in which electrical energy is converted into chemical energy. ELECTROLYSIS The chemical process in which electrical energy is converted to chemical energy OR the use of electrical energy to produce a chemical change.

ELECTROLYTIC CELL

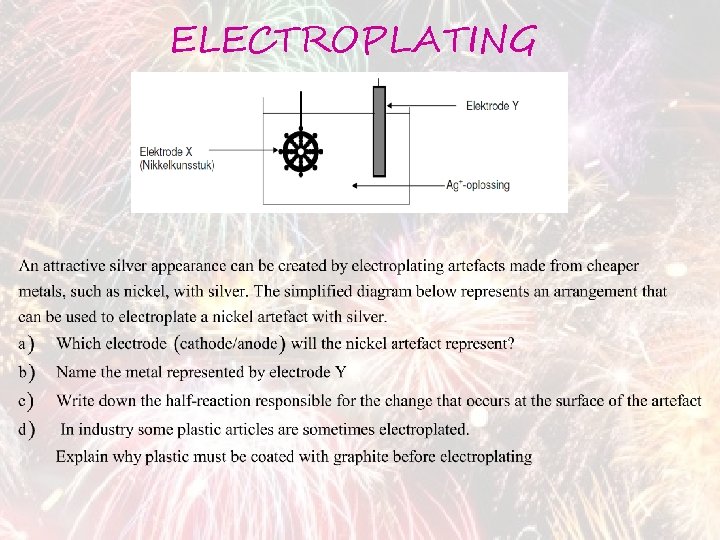
ELECTROPLATING

ELECTROPLATING

REFINING OF COPPER

REFINING OF COPPER

CHLOOR-ALKALI INDUSTRY

CHLOOR-ALKALI INDUSTRY

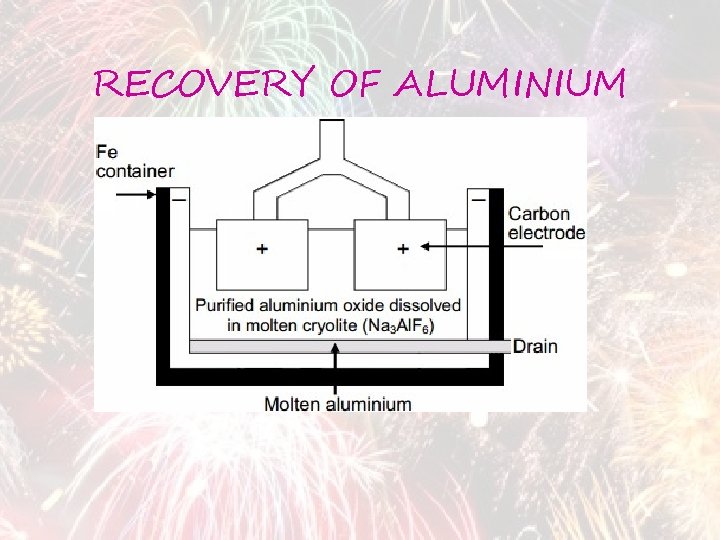
RECOVERY OF ALUMINIUM

RECOVERY OF ALUMINIUM

Oxidize Anode negative Reduce Cathode positive

Wants to oxidize ‘upside down’ Wants to reduce ‘rightside up’
 Passive sentence
Passive sentence Oxide thickness color chart
Oxide thickness color chart Normal loss treatment in process costing
Normal loss treatment in process costing Coupon technology
Coupon technology Diagonal rule electrochemistry
Diagonal rule electrochemistry E cell formula
E cell formula What is electrochemistry?
What is electrochemistry? Spontaneity electrochemistry
Spontaneity electrochemistry Fundamentals of electrochemistry
Fundamentals of electrochemistry Electrolytic cell khan academy
Electrolytic cell khan academy What is electrochemistry
What is electrochemistry Junction potential
Junction potential Ap chem electrochemistry
Ap chem electrochemistry Electrons flow from anode to cathode
Electrons flow from anode to cathode Electrochemistry balancing equations
Electrochemistry balancing equations What is polarization in electrochemistry
What is polarization in electrochemistry Chemistry
Chemistry Electrochemistry balancing equations
Electrochemistry balancing equations Electroanalytical techniques
Electroanalytical techniques Oxidation or reduction
Oxidation or reduction Electrochemistry
Electrochemistry Aee cd20f
Aee cd20f What is electrochemistry in chemistry
What is electrochemistry in chemistry Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Oxidation and reduction in galvanic cells
Oxidation and reduction in galvanic cells Analytical electrochemistry
Analytical electrochemistry Electrochemistry lesson
Electrochemistry lesson Mass transport electrochemistry
Mass transport electrochemistry Electrochemistry stoichiometry
Electrochemistry stoichiometry