Electrochemistry Lesson 4 Using Oxidation Numbers Using Oxidation

Electrochemistry Lesson 4 Using Oxidation Numbers
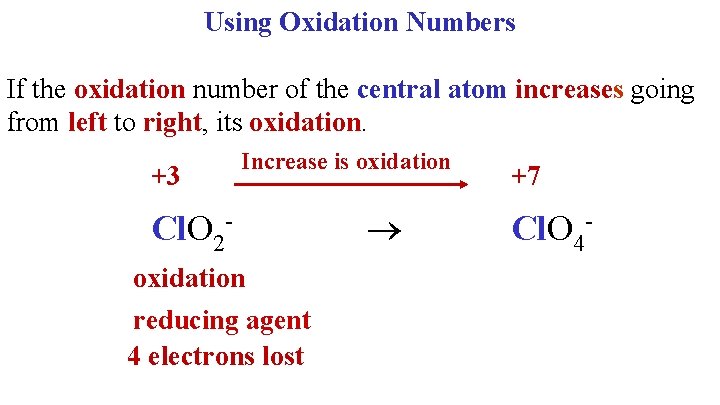
Using Oxidation Numbers If the oxidation number of the central atom increases going from left to right, its oxidation. +3 Increase is oxidation Cl. O 2 oxidation reducing agent 4 electrons lost +7 Cl. O 4 -

Using Oxidation Numbers If the oxidation number of the central atom decreases going from left to right, its reduction. +5 decrease is reduction NO 3 - reduction 2 electrons gained oxidizing agent +3 HNO 2
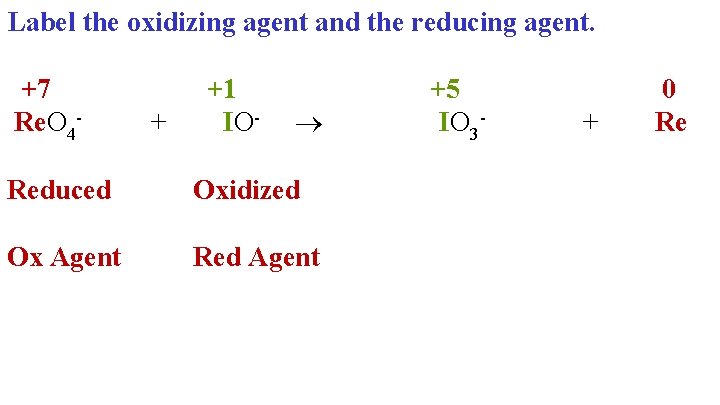
Label the oxidizing agent and the reducing agent. +7 +1 +5 Re. O 4+ IO- IO 3+ Reduced Oxidized Ox Agent Red Agent 0 Re
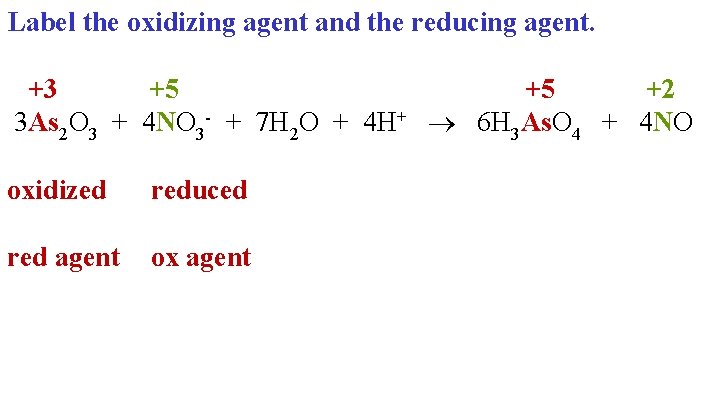
Label the oxidizing agent and the reducing agent. +3 +5 +5 +2 3 As 2 O 3 + 4 NO 3 - + 7 H 2 O + 4 H+ 6 H 3 As. O 4 + 4 NO oxidized reduced red agent ox agent

Predicting Spontaneous Reactions Using the Standard Reduction Chart Does Au 3+ react with Cl-? If it does write the spontaneous reaction.

The top reaction is written forward- reduction The bottom reaction is written in reverse- oxidation 3+ + 3 e- Au ) (Au 2 (s) - Cl (2 Cl 3 2(g) + 2 e-) 2 Au 3+ + 6 Cl- 3 Cl 2(g) + 2 Au(s)
Does Ag+ react with Br-?
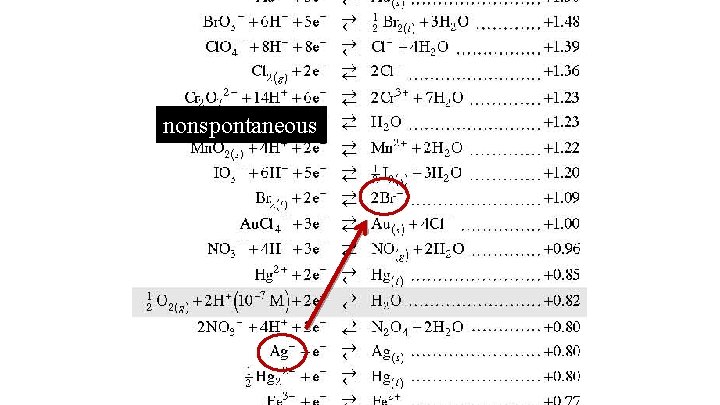
nonspontaneous
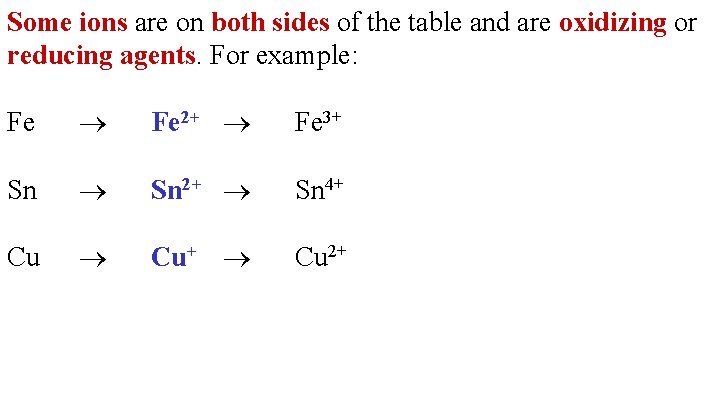
Some ions are on both sides of the table and are oxidizing or reducing agents. For example: Fe 2+ Fe 3+ Sn 2+ Sn 4+ Cu+ Cu 2+

Does Sn 2+ react with Cr?
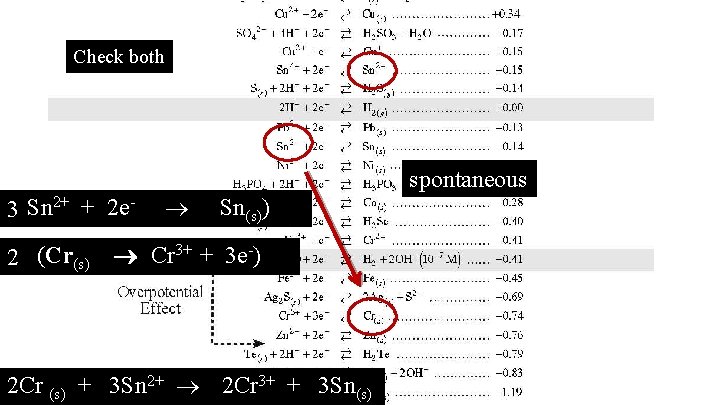
Check both spontaneous (Sn 3 2+ + 2 e- Sn(s)) 3+ + 3 e-) (Cr Cr 2 (s) 2 Cr (s) + 3 Sn 2+ 2 Cr 3+ + 3 Sn(s)

Can you keep HCl in a Cu container? Explain!

nonspontaneous

Can you keep HCl in a Cu container? Explain! Yes, nonspontaneous. H+ is a weaker oxidizing agent than Cu 2+ and Cu+.

Can you keep HCl in a Zn container? Explain!
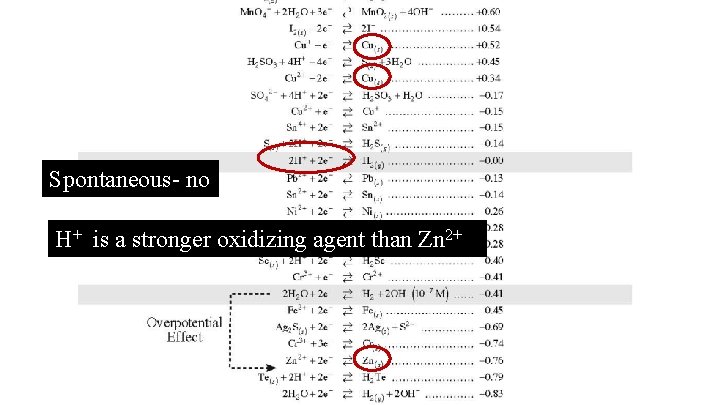
Spontaneous- no H+ is a stronger oxidizing agent than Zn 2+

Write the spontaneous reaction. 2 H+ + 2 e- H 2(g) Zn(s) Zn 2+ + 2 e 2 H+ + Zn(s) H 2(g) + Zn 2+

Can you keep HNO 3 in a Au container? Explain!


Can you keep HNO 3 in a Au container? Explain! Yes, nonspontaneous. HNO 3 is a weaker oxidizing agent than Au 3+.

Can you keep HNO 3 in a Cu container? Explain!

Take the strongest ox agent- higher Take the strongest red agent- lower
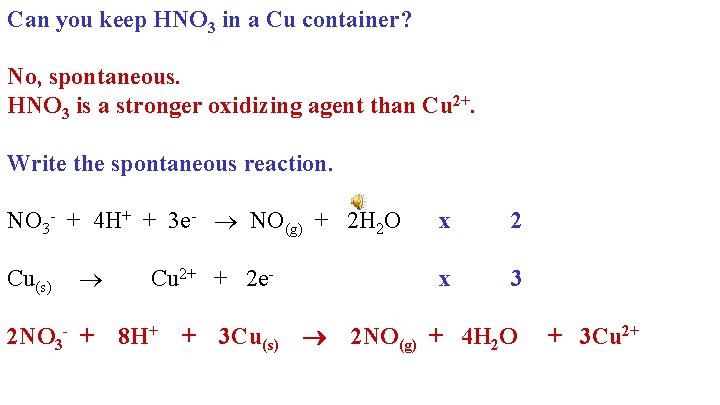
Can you keep HNO 3 in a Cu container? No, spontaneous. HNO 3 is a stronger oxidizing agent than Cu 2+. Write the spontaneous reaction. NO 3 - + 4 H+ + 3 e- NO(g) + 2 H 2 O Cu(s) 2 NO 3 - + Cu 2+ + 2 e 8 H+ + 3 Cu(s) x 2 x 3 2 NO(g) + 4 H 2 O + 3 Cu 2+
- Slides: 24