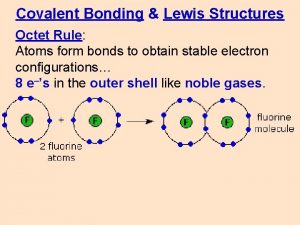
Review Chemical Reactivity Octet rule atoms tend to


























- Slides: 62


Review Chemical Reactivity Octet rule ___________________ atoms tend to gain, lose or share electrons to try and have eight electrons in their outer shell. Noble gases are non-reactive because they have 8 valence electrons.

IONIC BONDING

• Its all about “I” • Bonding by gaining or losing electrons to achieve a full outer shell

Valence Electrons in the outer most shell We use these to help with bonding

Definitions Ion – charged atom (atom that has gained/lost electron and has a positive or negative charge) Cation – ion with positive charge Anion – ion with negative charge

Ions An ion is a atom that has gained or lost one or more electrons and has a positive or negative charge. Atoms gain or lose electrons in order to achieve the octet rule (8 valence electrons)

Ions NOTE – when you see a (+), the atom is LOSING an electron. NOTE – when you see a (-), the atom is GAINING an electron

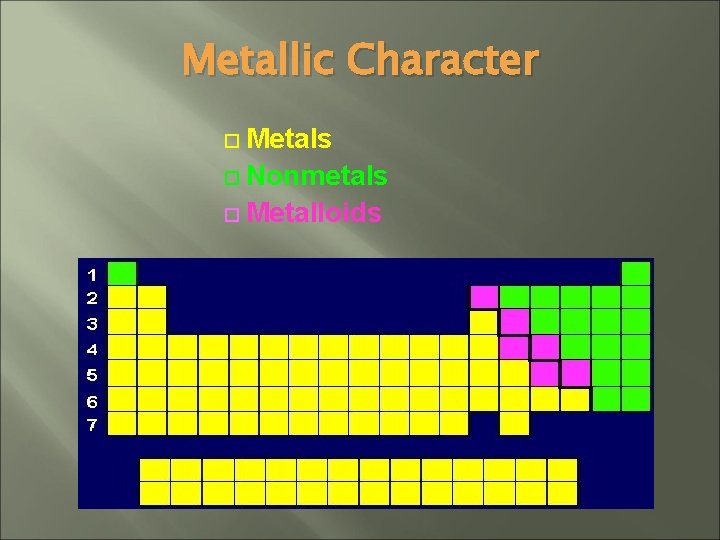
Metallic Character Metals Nonmetals Metalloids

Metal Elements Nearly all metals form cations. Mg has 2 valence electrons. It is much easier to lose two electrons than gain six electrons. Mg 2+ …. . cation

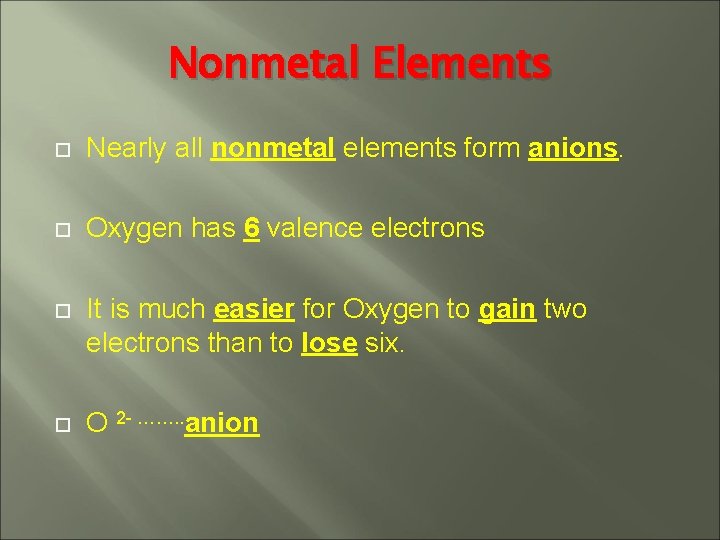
Nonmetal Elements Nearly all nonmetal elements form anions. Oxygen has 6 valence electrons It is much easier for Oxygen to gain two electrons than to lose six. O 2 - ……. . anion

Ion Names Naming a Cation (positive ions, atoms that lose electrons) Simply the name of the element Example: Na+ - sodium ion Mg 2+ - Magnesium ion

Ion Names Naming an Anion – (negative ions, atoms that gains electrons) The element name ends in –ide. Example: Cl- - chloride ion O 2 - - oxide ion

Ionic Bonding The force of attraction between a positive charge and negative charge creates the ionic bond. Ex: Wants to be neutral…Sodium ion (Na+) has a +1 charge and Chloride ion (Cl-) has a -1 charge. Sodium Chloride…table salt

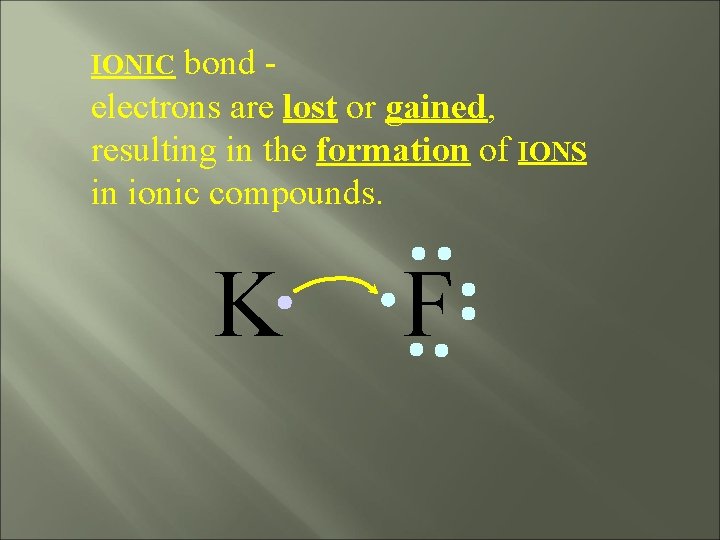
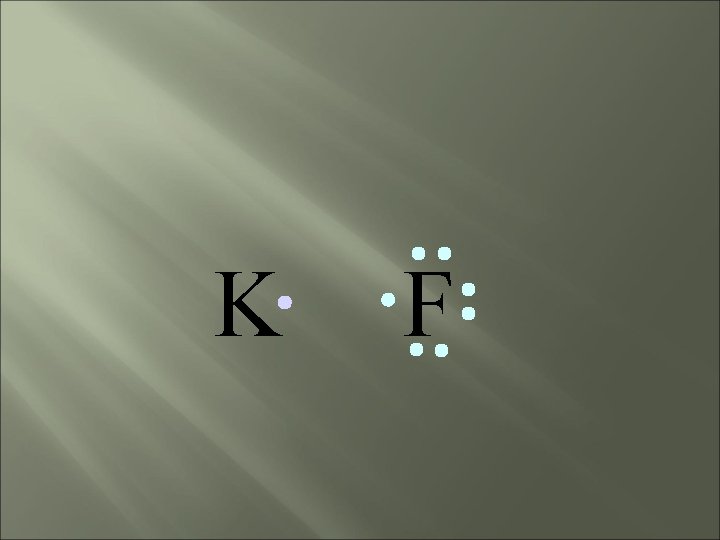
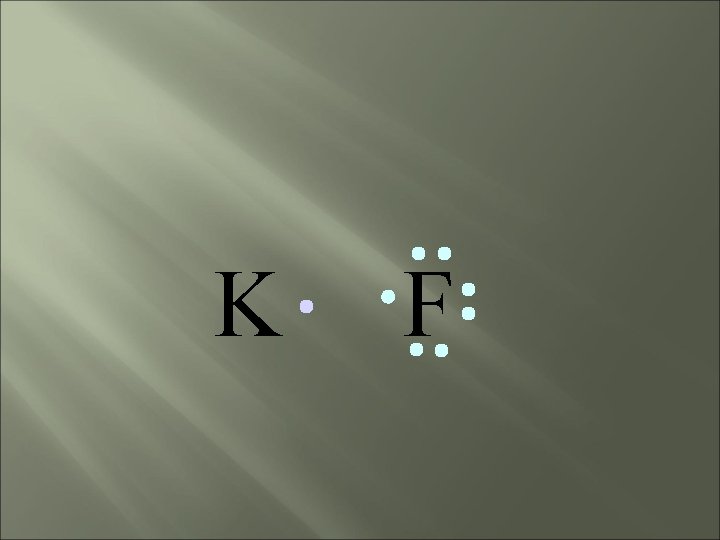
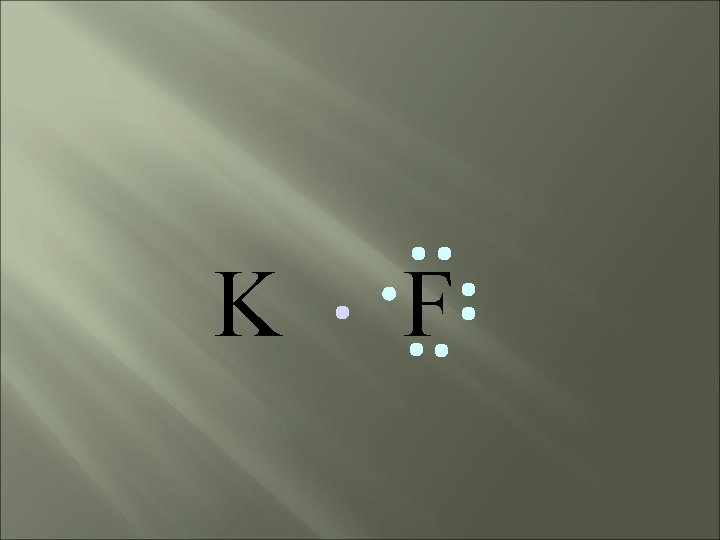
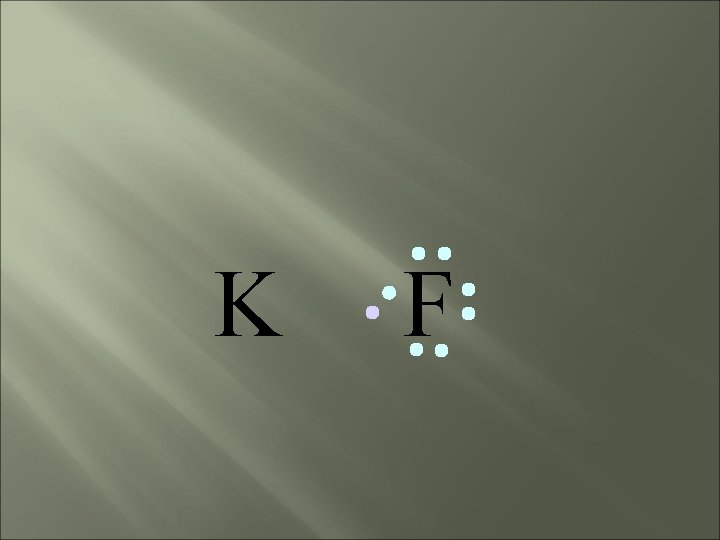
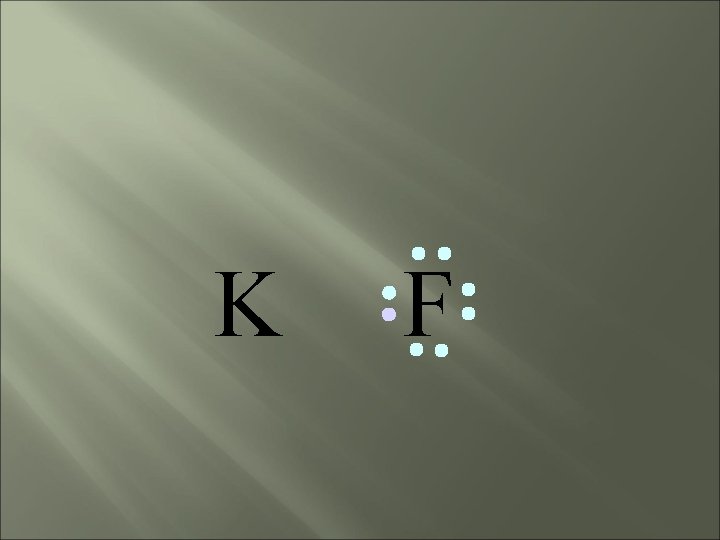
bond electrons are lost or gained, resulting in the formation of IONS in ionic compounds. IONIC K F

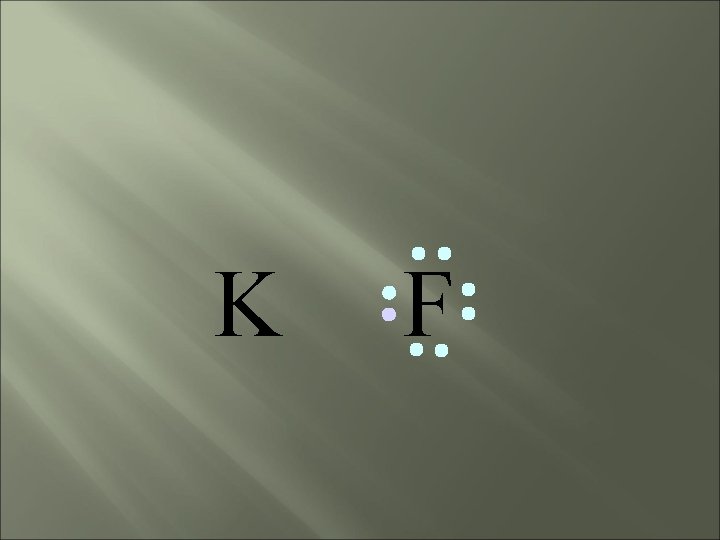
K F

K F

K F

K F

K F

K F


Ionic Compound Names The cation goes first, then the anion Remember when naming an Anion – (atom that gains electrons) it will end in ide Example: Na. Cl Sodium Chloride Mg. O Magnesium Oxide

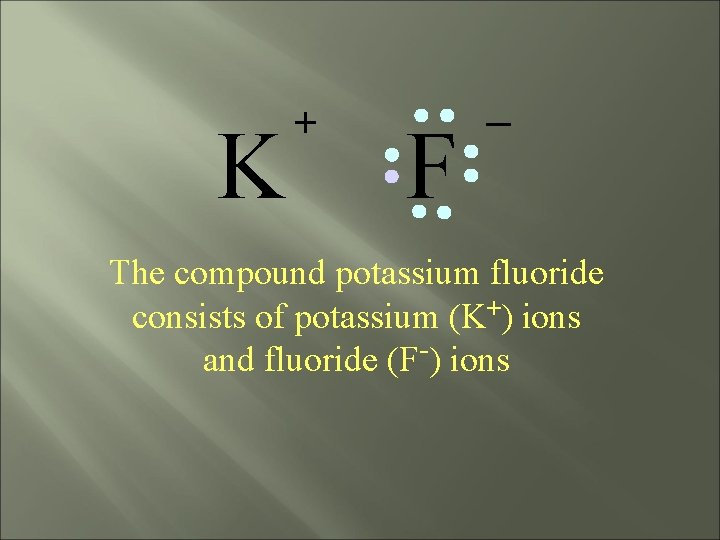
K + F _ The compound potassium fluoride consists of potassium (K+) ions and fluoride (F-) ions

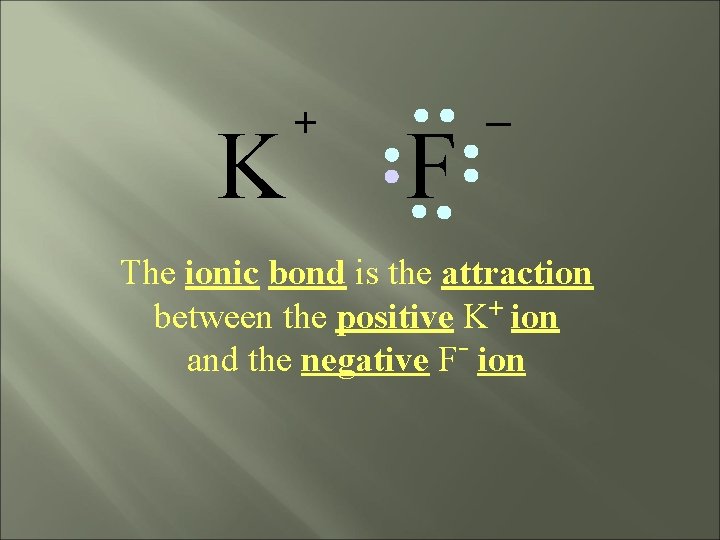
K + F _ The ionic bond is the attraction between the positive K+ ion and the negative F- ion

Covalent Bonds

Covalent Bonding by sharing electrons to achieve a full outer shell

• In covalent bonding, atoms still want to achieve a noble gas configuration (the octet rule). • But rather than losing or gaining electrons, atoms now share an electron pair. • The shared electron pair is called a bonding pair

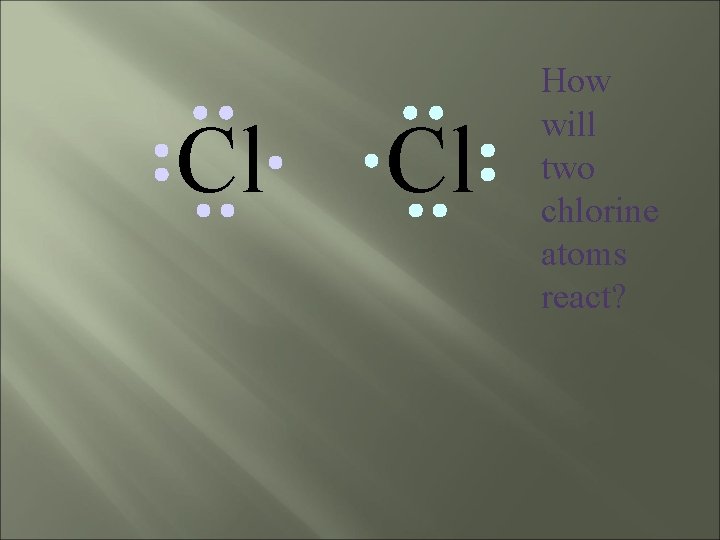
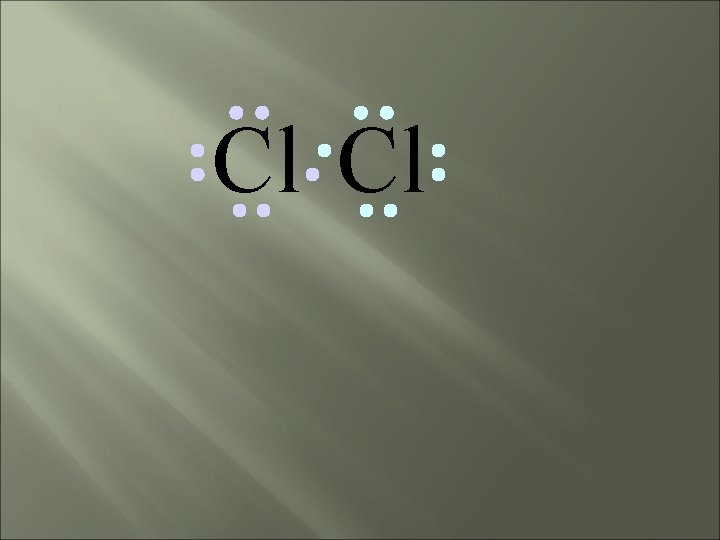
Chlorine forms a covalent bond with itself Cl 2

Cl Cl How will two chlorine atoms react?

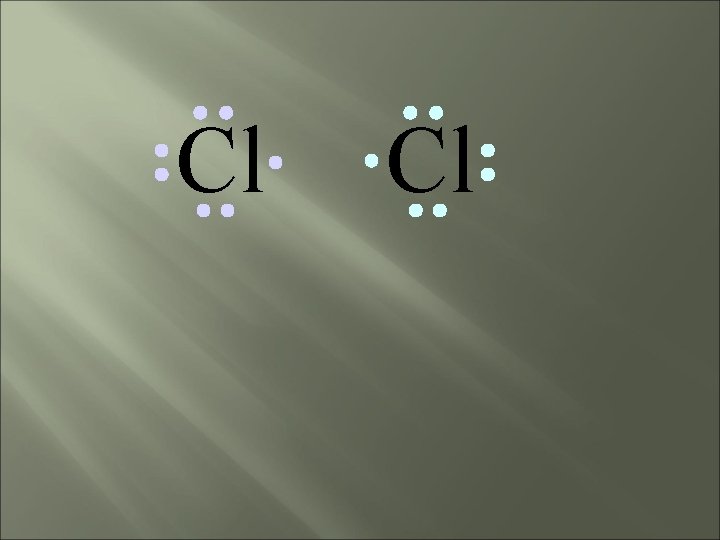
Cl Cl Each chlorine atom wants to gain one electron to achieve an octet

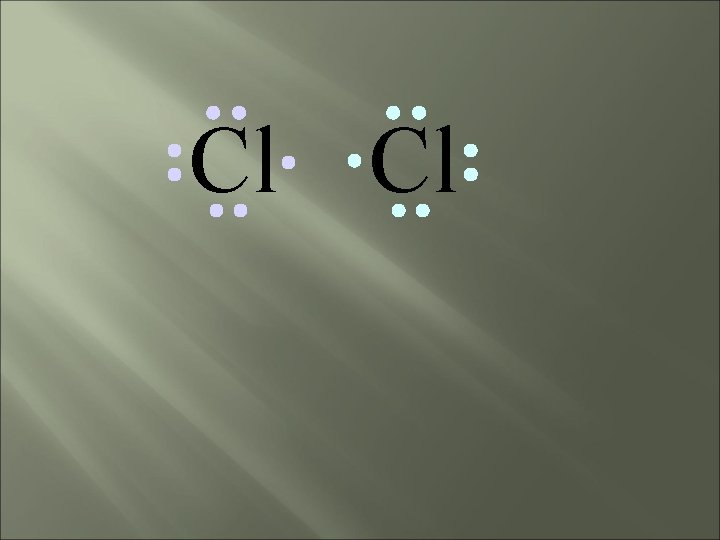
Cl Cl Neither atom will give up an electron – What’s the solution – what can they do to achieve an octet?

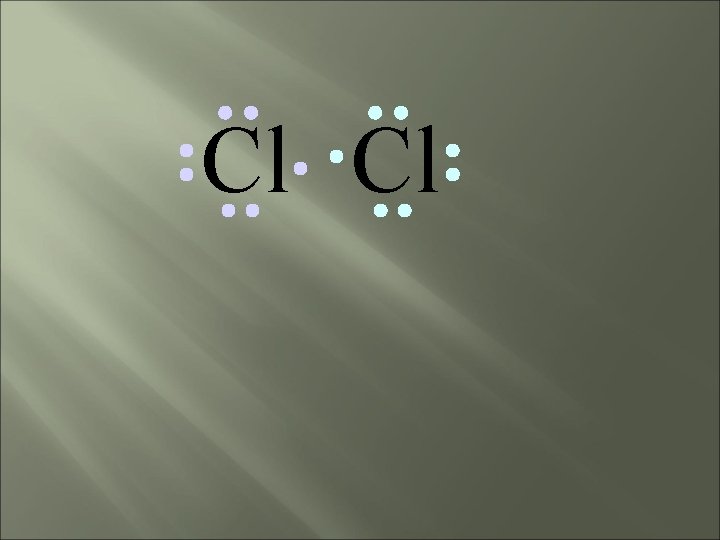
Cl Cl

Cl Cl

Cl Cl

Cl Cl

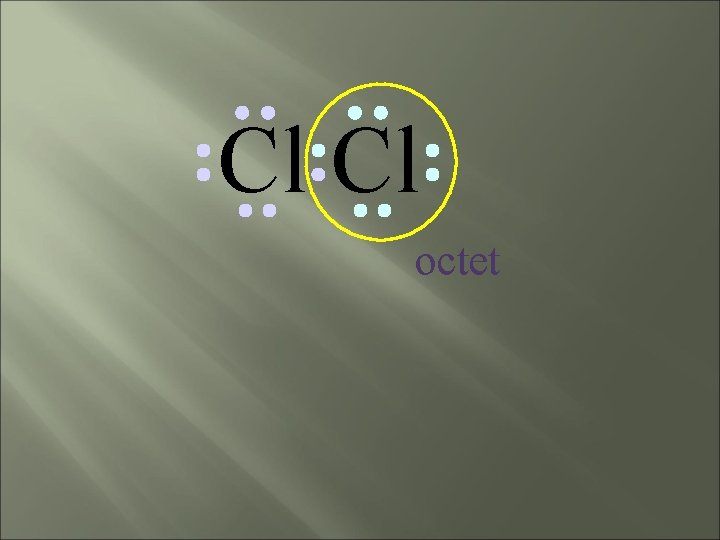
Cl Cl octet

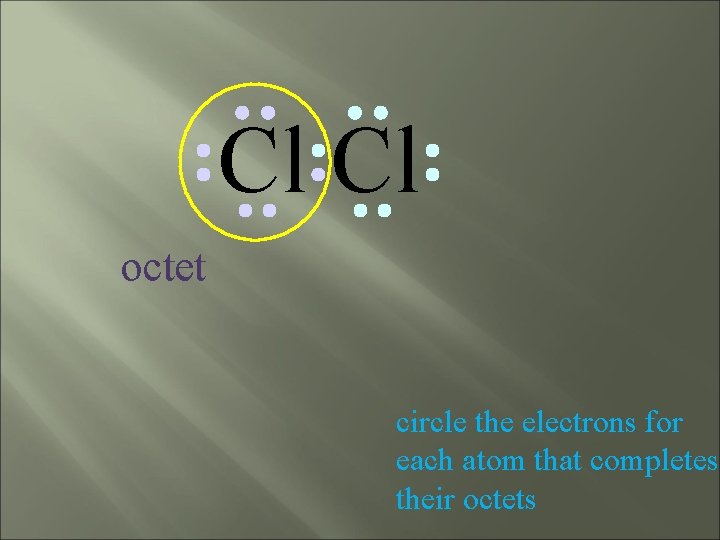
Cl Cl octet circle the electrons for each atom that completes their octets

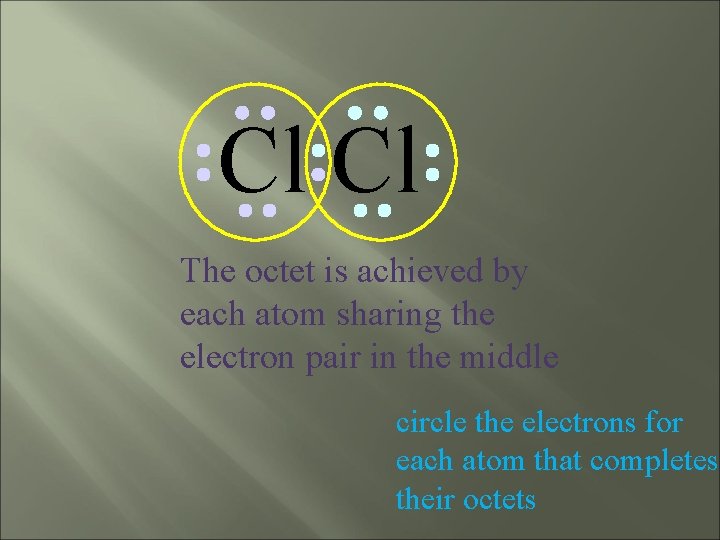
Cl Cl The octet is achieved by each atom sharing the electron pair in the middle circle the electrons for each atom that completes their octets

Cl Cl The octet is achieved by each atom sharing the electron pair in the middle circle the electrons for each atom that completes their octets

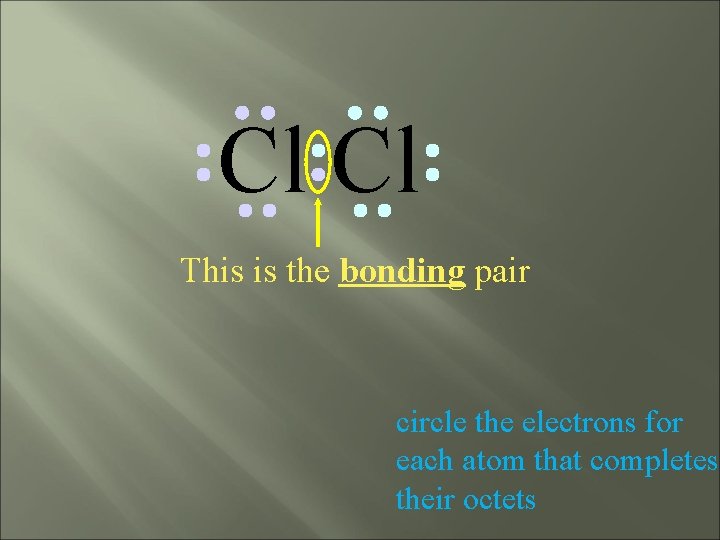
Cl Cl This is the bonding pair circle the electrons for each atom that completes their octets

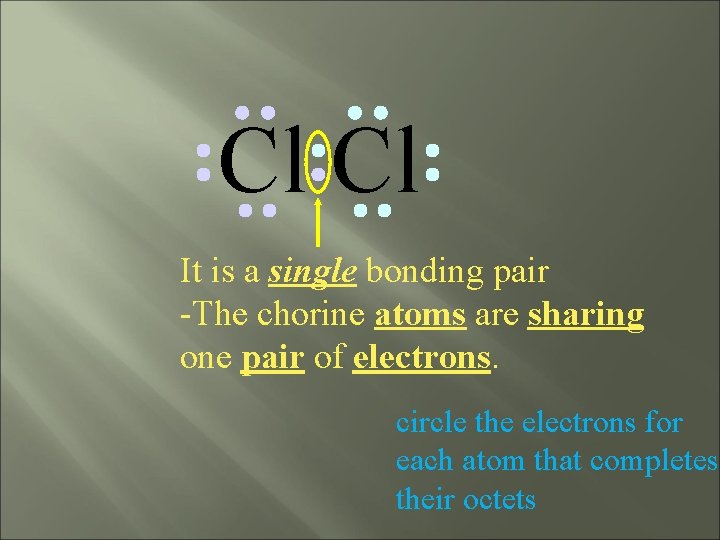
Cl Cl It is a single bonding pair -The chorine atoms are sharing one pair of electrons. circle the electrons for each atom that completes their octets

Cl Cl It is called a SINGLE BOND circle the electrons for each atom that completes their octets

Cl Cl Single bonds are abbreviated with a dash circle the electrons for each atom that completes their octets

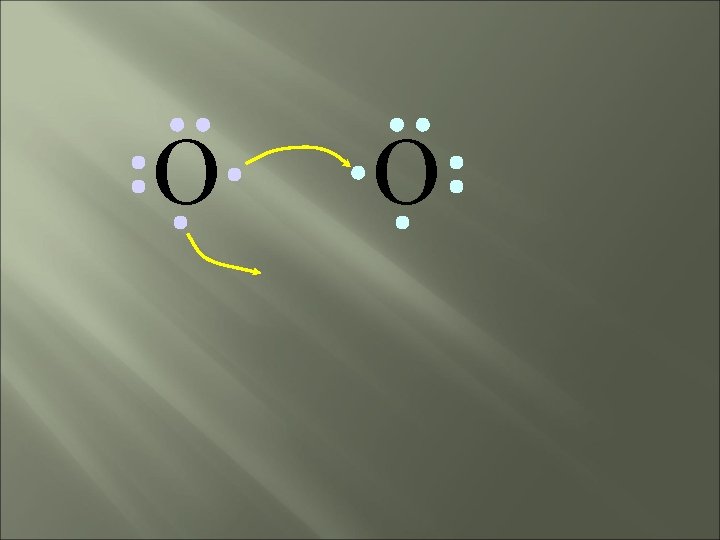
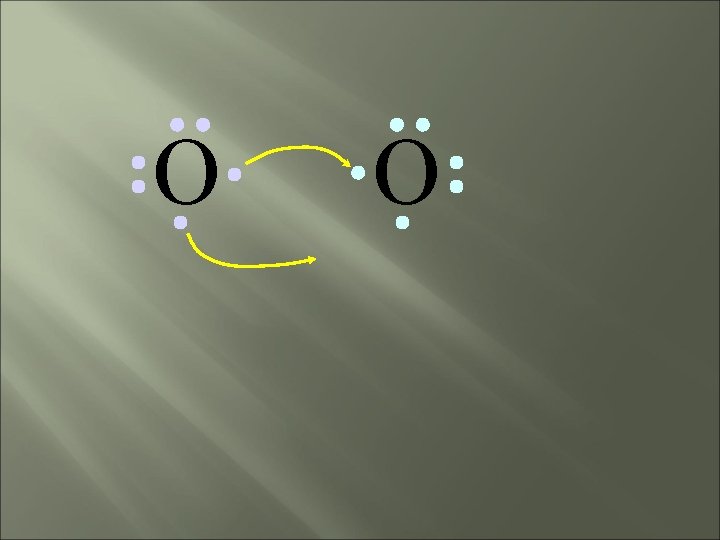
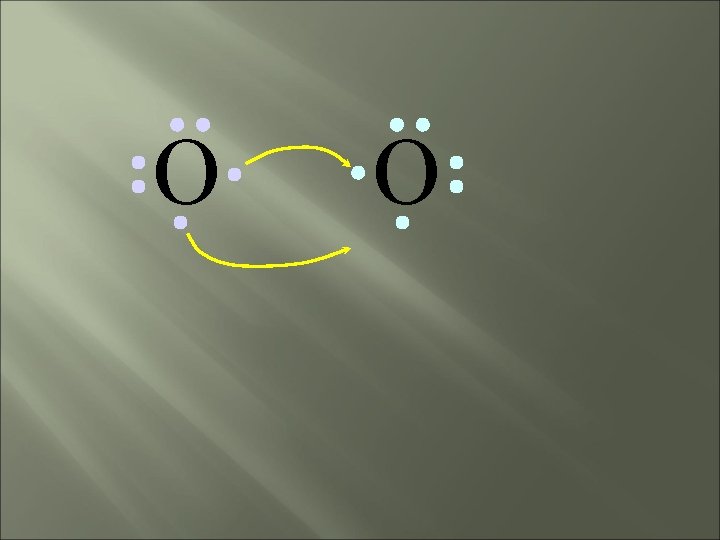
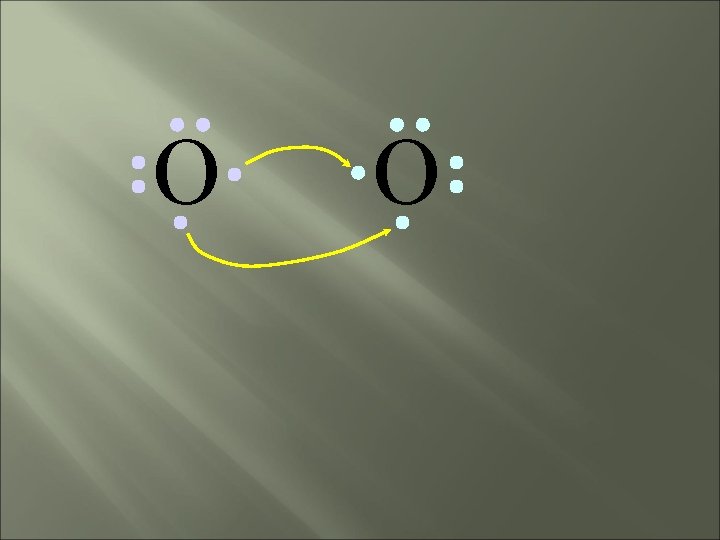
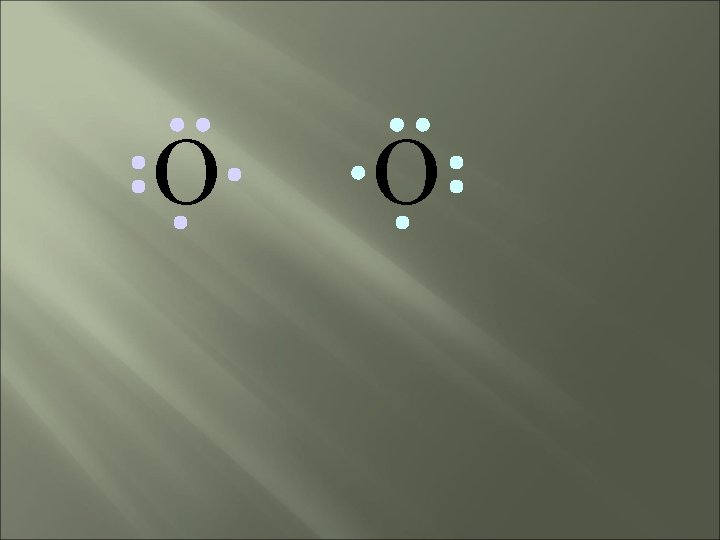
O O How will two oxygen atoms bond?

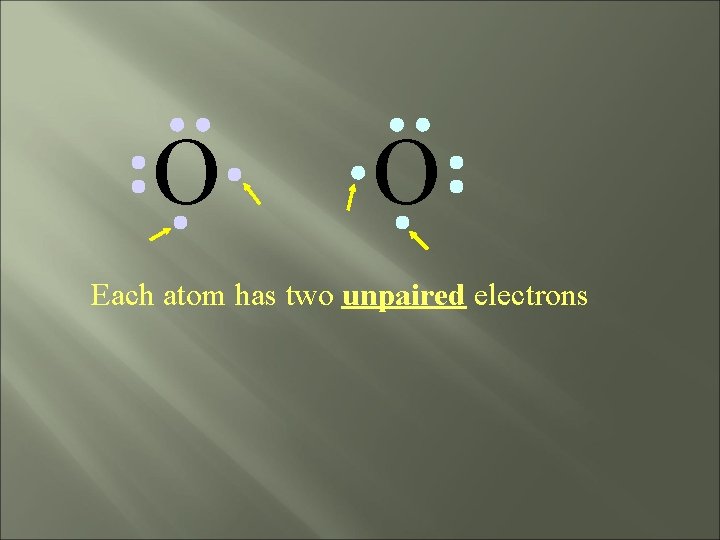
O O Each atom has two unpaired electrons

O O

O O

O O

O O

O O

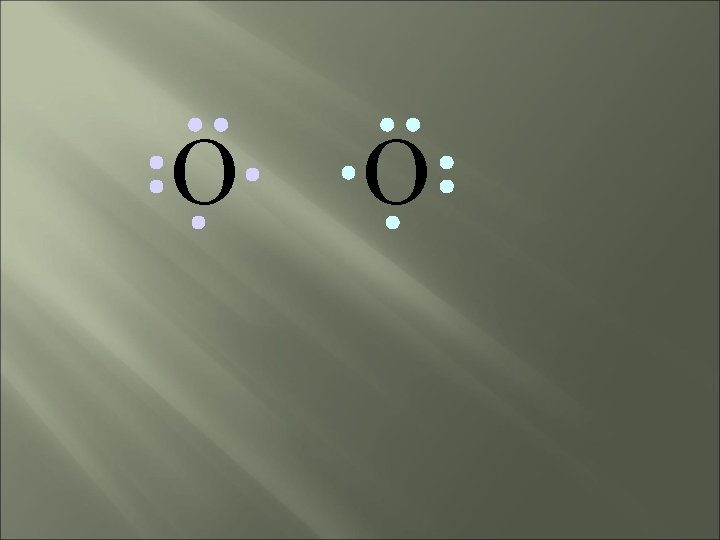
O O Both atoms want to gain two electrons.

O O

O O

O O

O O

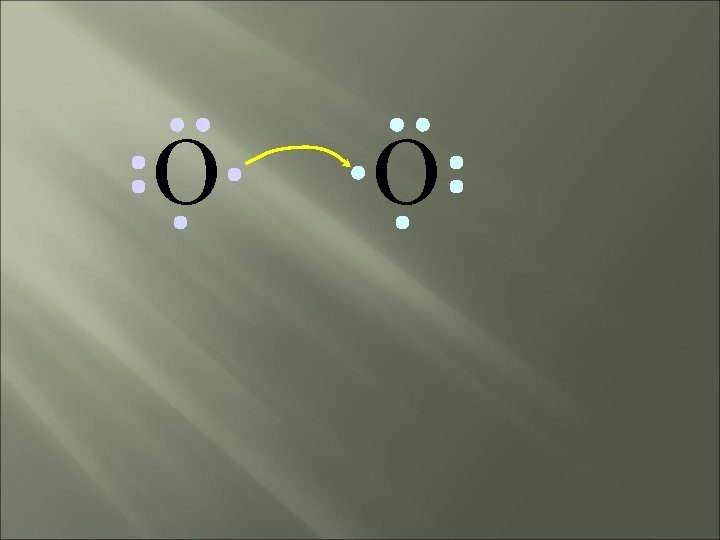
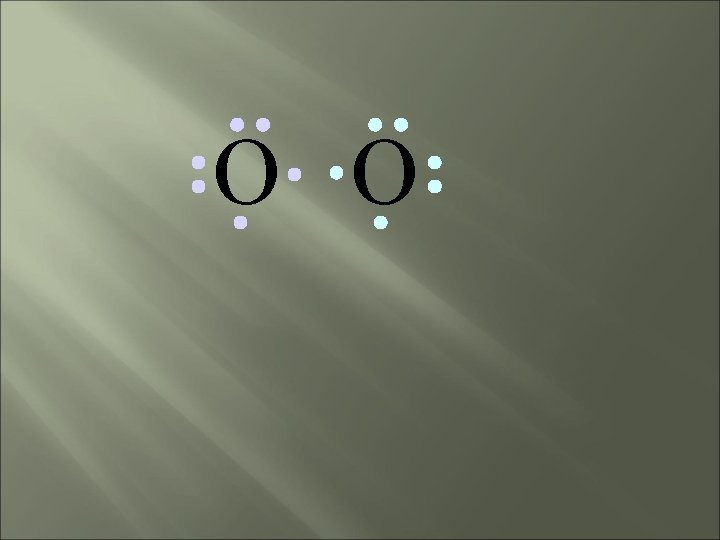
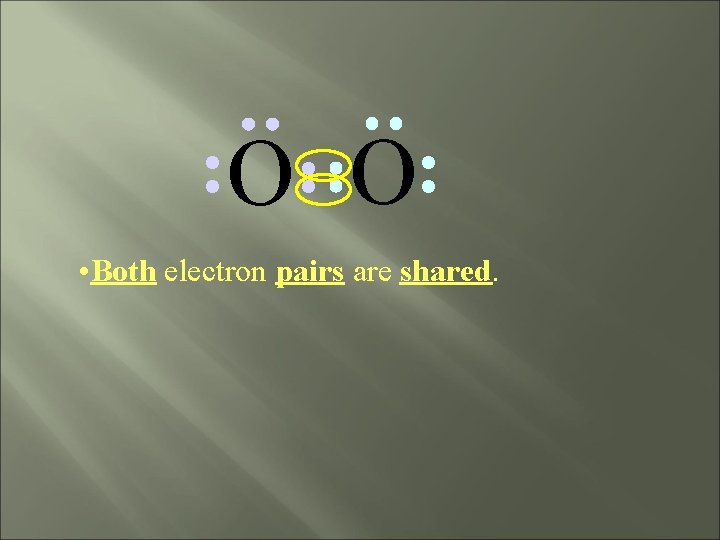
O O • Both electron pairs are shared.

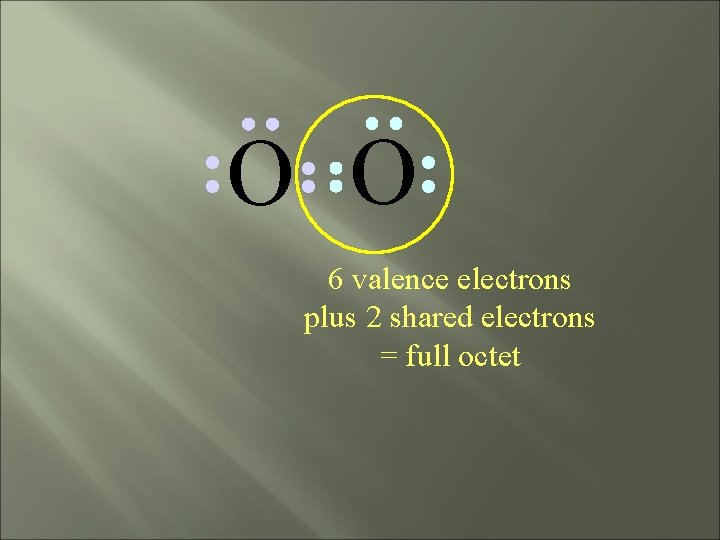
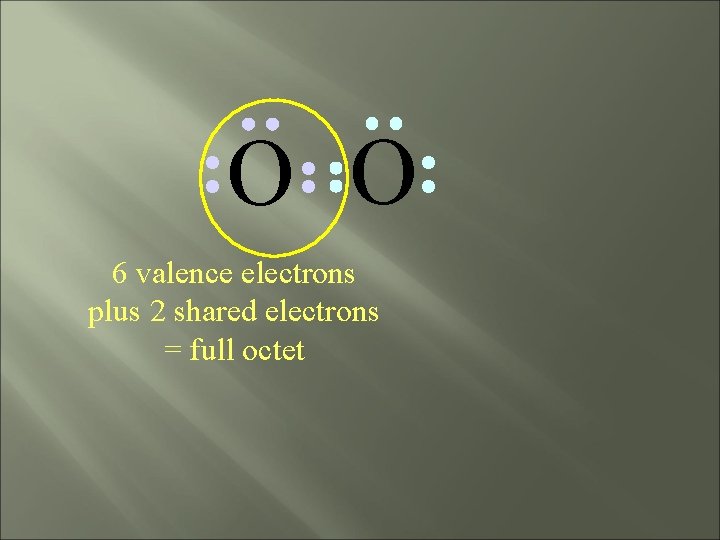
O O 6 valence electrons plus 2 shared electrons = full octet

O O 6 valence electrons plus 2 shared electrons = full octet

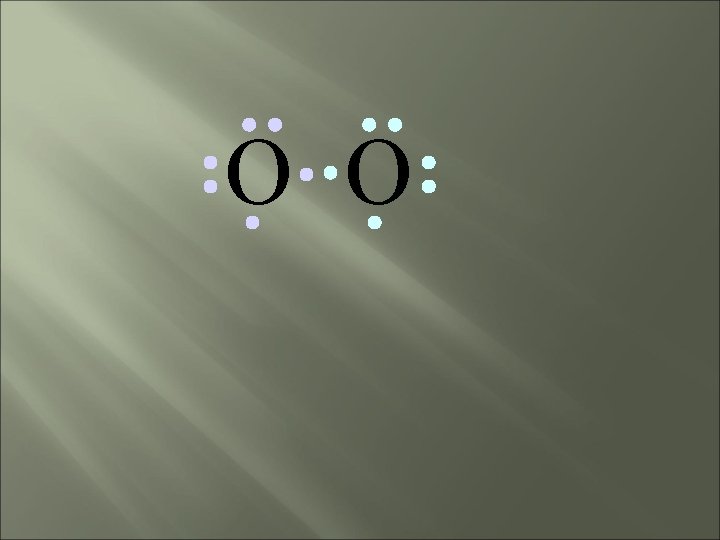
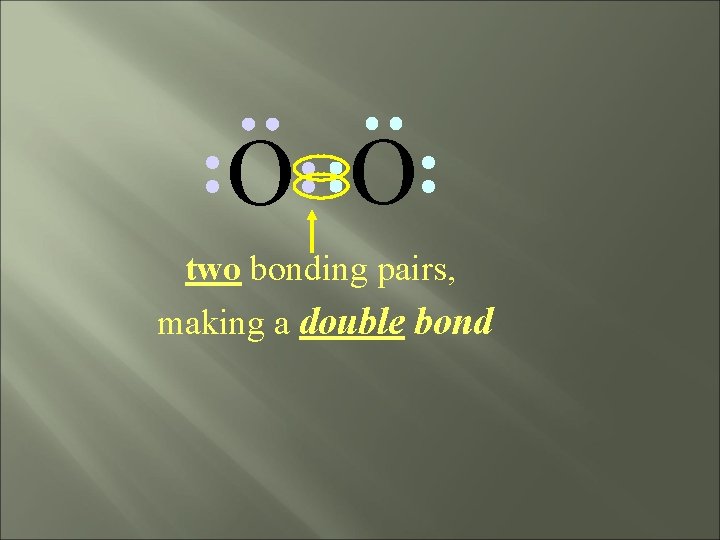
O O two bonding pairs, making a double bond

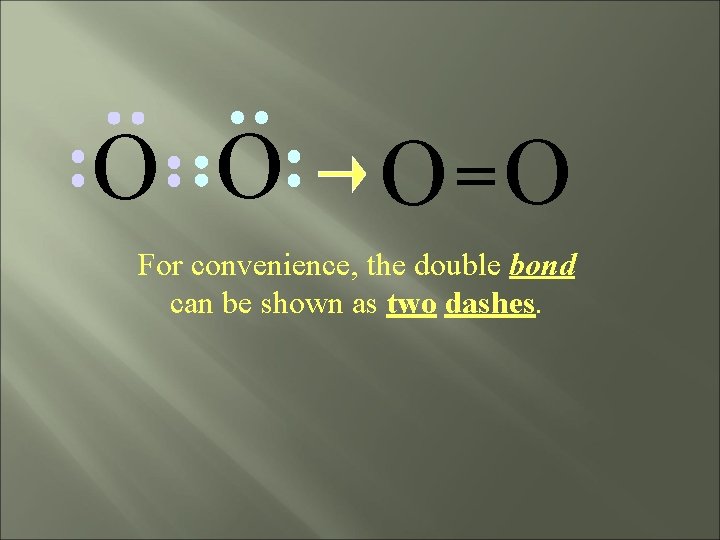
O O O =O For convenience, the double bond can be shown as two dashes.

Naming Covalent Bonds Possible Quiz on line http: //www. mpdocker. demon. co. uk/as_a 2/topics/ionic_and_co valent_bonding/quiz_2. html
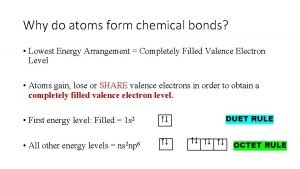
 Why do atoms form chemical bonds? *
Why do atoms form chemical bonds? * Valence electrons of xenon
Valence electrons of xenon What atoms can have an expanded octet
What atoms can have an expanded octet What is the oxidation number of lithium
What is the oxidation number of lithium Periodic table of elements regents
Periodic table of elements regents The octet rule states that
The octet rule states that What is octet rule
What is octet rule The central atom in ________ violates the octet rule.
The central atom in ________ violates the octet rule. Vsepr notation
Vsepr notation Atoms form
Atoms form Exceptions to the octet rule
Exceptions to the octet rule Bent molecular shape
Bent molecular shape The octet rule states that
The octet rule states that Mg octet rule
Mg octet rule Honc rule
Honc rule Octet rule
Octet rule Hcn lewis structure
Hcn lewis structure Noble gases octet rule
Noble gases octet rule Non metallic examples
Non metallic examples Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Chapter 7 review modern chemistry answers
Chapter 7 review modern chemistry answers Reactivity of alkali metal
Reactivity of alkali metal What's the difference between solution and suspension
What's the difference between solution and suspension Rearranging atoms answer key
Rearranging atoms answer key How many atoms
How many atoms Chemical change atoms
Chemical change atoms Chemical bonds form when atoms *
Chemical bonds form when atoms * Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Why do atoms form bonds?
Why do atoms form bonds? In chemical reactions atoms are rearranged
In chemical reactions atoms are rearranged Chapter 5 arrangement of electrons
Chapter 5 arrangement of electrons Octave and sestet are part of
Octave and sestet are part of Ominous octet
Ominous octet Group polarization
Group polarization Shakespeare 57 sonnet
Shakespeare 57 sonnet Octet red 96
Octet red 96 Modern organizations tend to
Modern organizations tend to Ominous octet
Ominous octet Positive subjective experiences tend to be
Positive subjective experiences tend to be Important lewis structures
Important lewis structures Extended octet
Extended octet Bicultural couples tend to demonstrate extremes in
Bicultural couples tend to demonstrate extremes in Octet bli
Octet bli Doublet non liant
Doublet non liant First octet of ip address
First octet of ip address Systems in nature tend to undergo changes toward
Systems in nature tend to undergo changes toward Moodle umft login
Moodle umft login Children tend to learn english
Children tend to learn english Octet kinetics
Octet kinetics Octet system
Octet system Deaf tend theirs
Deaf tend theirs First octet of ip address
First octet of ip address N2o dot and cross
N2o dot and cross Number of bits borrowed
Number of bits borrowed Atoms vs elements
Atoms vs elements Shakespeare's comedies tend to end with a
Shakespeare's comedies tend to end with a Ominous octet diabetes
Ominous octet diabetes Generally restful like a horizontal the sky meets land
Generally restful like a horizontal the sky meets land Disque dur 1 peta
Disque dur 1 peta Ominous octet
Ominous octet