Lewis Structures Molecular Geometries Lewis Dot Diagrams Represent




- Slides: 16

Lewis Structures & Molecular Geometries

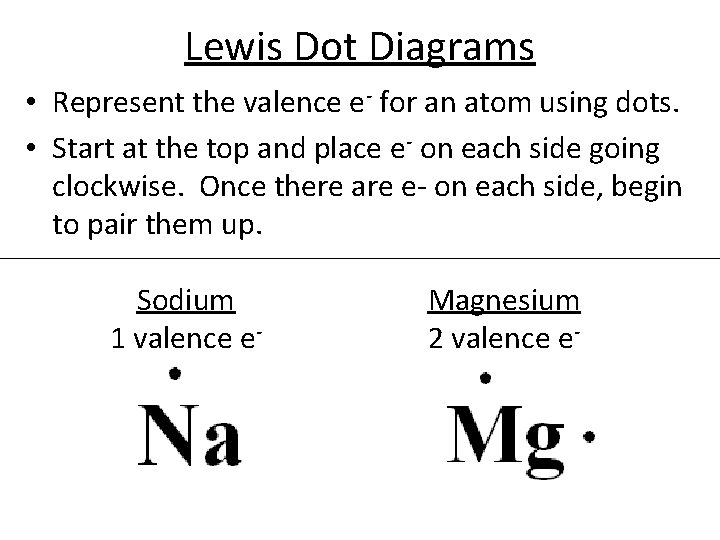
Lewis Dot Diagrams • Represent the valence e- for an atom using dots. • Start at the top and place e- on each side going clockwise. Once there are e- on each side, begin to pair them up. Sodium 1 valence e- Magnesium 2 valence e-

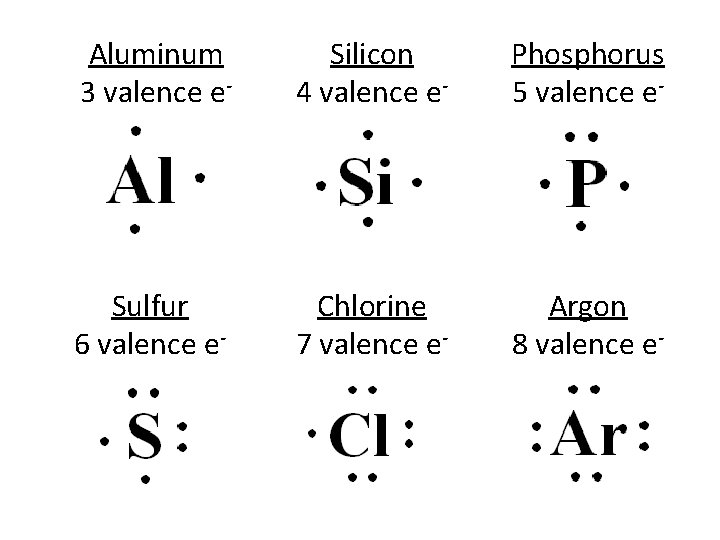
Aluminum 3 valence e- Silicon 4 valence e- Phosphorus 5 valence e- Sulfur 6 valence e- Chlorine 7 valence e- Argon 8 valence e-

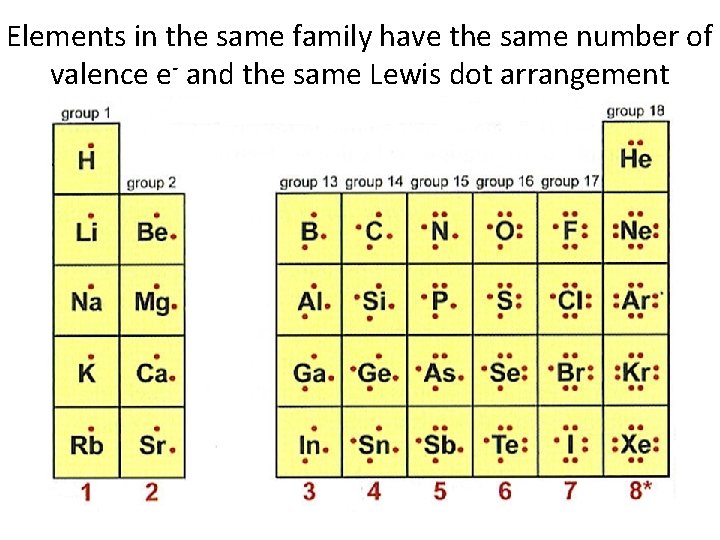
Elements in the same family have the same number of valence e- and the same Lewis dot arrangement

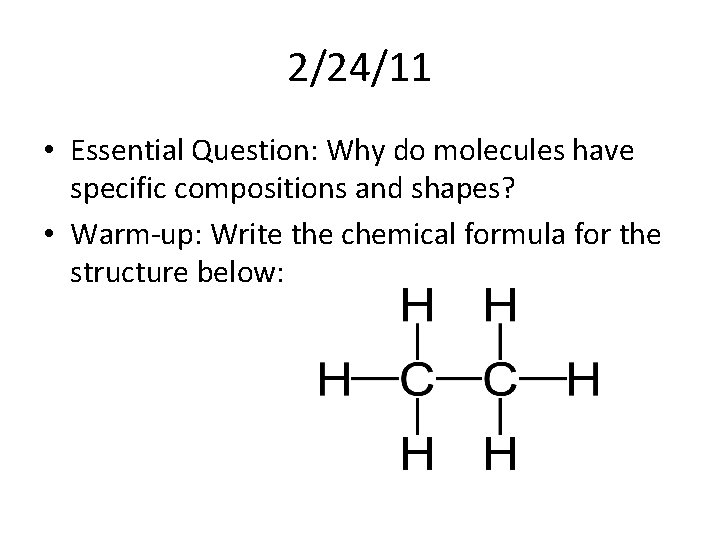
2/24/11 • Essential Question: Why do molecules have specific compositions and shapes? • Warm-up: Write the chemical formula for the structure below:

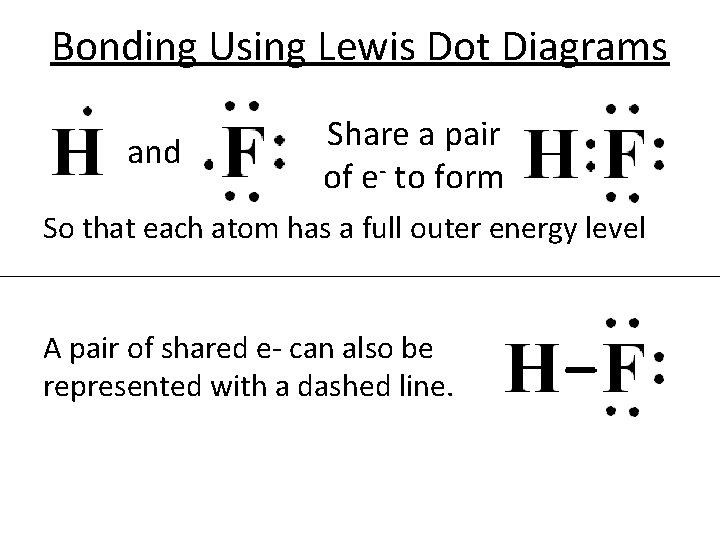
Bonding Using Lewis Dot Diagrams and Share a pair of e- to form So that each atom has a full outer energy level A pair of shared e- can also be represented with a dashed line.

Exceptions to the Octet Rule • In covalent bonds, atoms always share e- to reach a full valence shell of 8 valence e-…except… – Hydrogen only needs 2 e- in its outer energy level. – Boron only needs 6 e- in its outer energy level. – Some elements can form an expanded octet using empty d-orbitals to form bonds and have more than 8 valence e-.

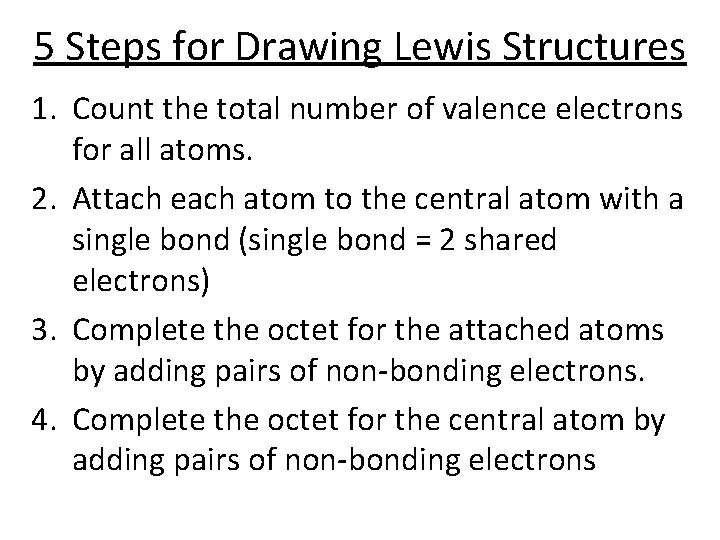
5 Steps for Drawing Lewis Structures 1. Count the total number of valence electrons for all atoms. 2. Attach each atom to the central atom with a single bond (single bond = 2 shared electrons) 3. Complete the octet for the attached atoms by adding pairs of non-bonding electrons. 4. Complete the octet for the central atom by adding pairs of non-bonding electrons

5. Count the total number of electrons in your structure and compare to step one. – If the number of e- is the same, it is correct. – If you used too many e-, add double bond(s) and check your total again (usually add one double bond for each two electrons that you are over the total). – If there are extra electrons left over, add them as non-bonding electrons on the central atom. This is called an expanded octet.

Warm-Up 2/25/11 • Complete Part 6 of Activity 2 B.

Valence Shell Electron Pair Repulsion Theory • Abbreviated “VSEPR” • Pairs of e- around an atom repel each other and will form an arrangement that minimizes this repulsion (i. e. spread as far apart from each other as possible). As a result, molecules tend to form predictable shapes. • Lone pairs of non-bonding e- have greater repulsion than bonded pairs of e-.

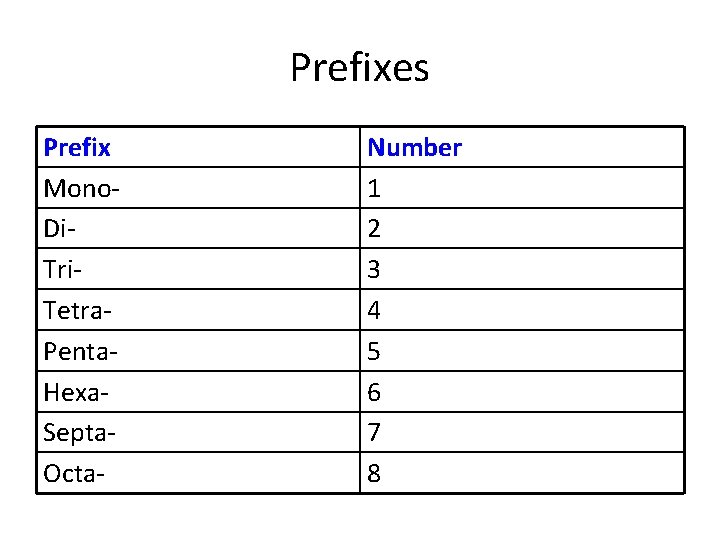
Prefixes Prefix Mono. Di. Tri. Tetra. Penta. Hexa. Septa. Octa- Number 1 2 3 4 5 6 7 8

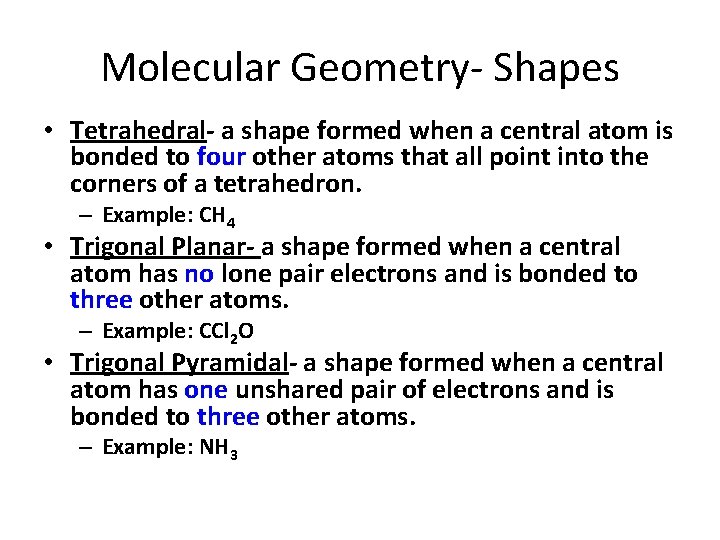
Molecular Geometry- Shapes • Tetrahedral- a shape formed when a central atom is bonded to four other atoms that all point into the corners of a tetrahedron. – Example: CH 4 • Trigonal Planar- a shape formed when a central atom has no lone pair electrons and is bonded to three other atoms. – Example: CCl 2 O • Trigonal Pyramidal- a shape formed when a central atom has one unshared pair of electrons and is bonded to three other atoms. – Example: NH 3

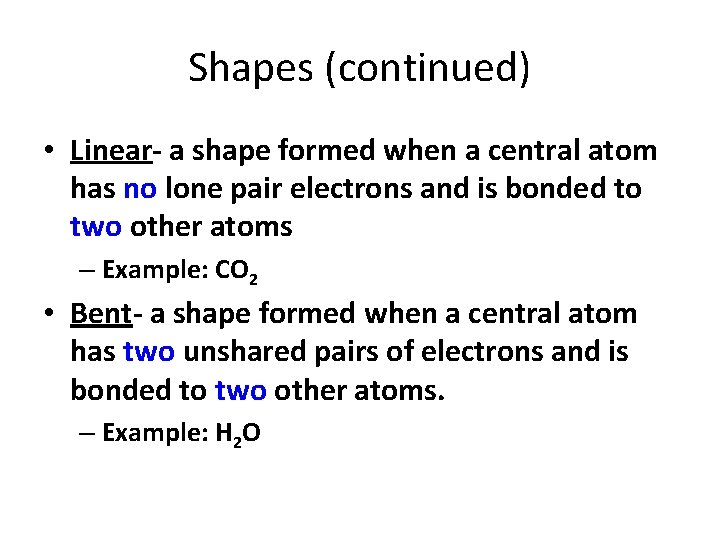
Shapes (continued) • Linear- a shape formed when a central atom has no lone pair electrons and is bonded to two other atoms – Example: CO 2 • Bent- a shape formed when a central atom has two unshared pairs of electrons and is bonded to two other atoms. – Example: H 2 O

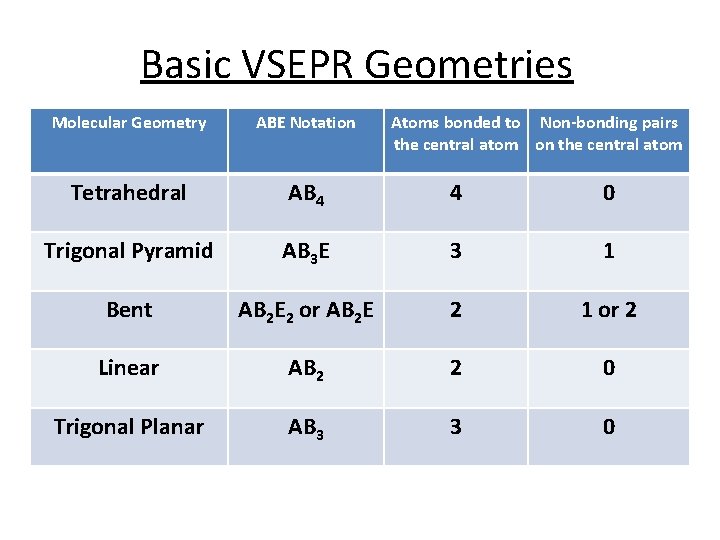
Basic VSEPR Geometries Molecular Geometry ABE Notation Atoms bonded to Non-bonding pairs the central atom on the central atom Tetrahedral AB 4 4 0 Trigonal Pyramid AB 3 E 3 1 Bent AB 2 E 2 or AB 2 E 2 1 or 2 Linear AB 2 2 0 Trigonal Planar AB 3 3 0

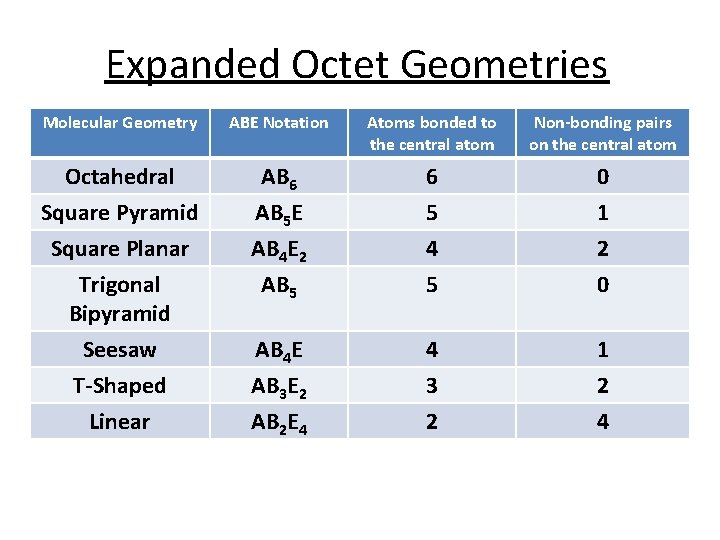
Expanded Octet Geometries Molecular Geometry ABE Notation Atoms bonded to the central atom Non-bonding pairs on the central atom Octahedral AB 6 6 0 Square Pyramid Square Planar Trigonal Bipyramid Seesaw AB 5 E AB 4 E 2 AB 5 5 4 5 1 2 0 AB 4 E 4 1 T-Shaped Linear AB 3 E 2 AB 2 E 4 3 2 2 4