Whats coming up Oct 25 Oct 27 Oct




- Slides: 31

What’s coming up? ? ? • • • Oct 25 Oct 27 Oct 29 Nov 1 Nov 3, 5 Nov 8, 10 Nov 12 Nov 15 Nov 17 The atmosphere, part 1 Midterm … No lecture The atmosphere, part 2 Light, blackbodies, Bohr Postulates of QM, p-in-a-box Hydrogen and multi – e atoms Multi-electron atoms Periodic properties Ch. 8 Ch. 9, 10 Ch. 10 • Nov 19 Valence-bond; Lewis structures Ch. 11 • • • VSEPR Hybrid orbitals; VSEPR MO theory bonding wrapup Review for exam Nov 22 Nov 24 Nov 26 Nov 29 Dec 1 Dec 2 Ch. 11, 12


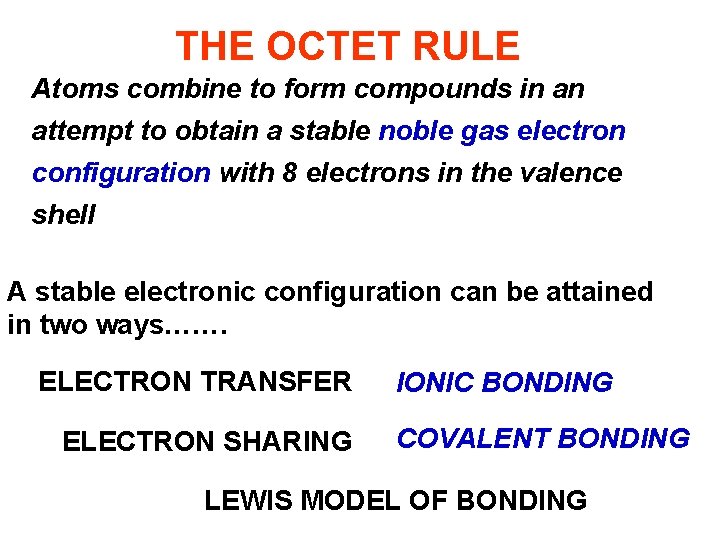
THE OCTET RULE Atoms combine to form compounds in an attempt to obtain a stable noble gas electron configuration with 8 electrons in the valence shell A stable electronic configuration can be attained in two ways……. ELECTRON TRANSFER ELECTRON SHARING IONIC BONDING COVALENT BONDING LEWIS MODEL OF BONDING

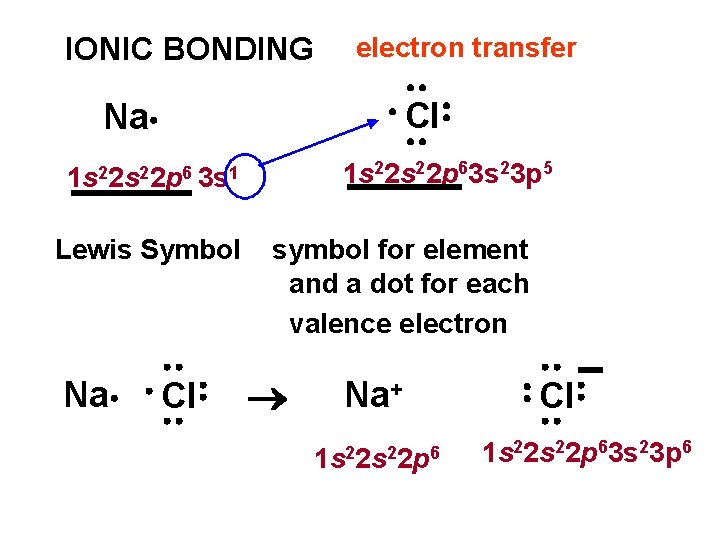
IONIC BONDING electron transfer Cl Na 1 s 22 p 63 s 23 p 5 1 s 22 p 6 3 s 1 Lewis Symbol Na Cl symbol for element and a dot for each valence electron Na+ 1 s 22 p 6 Cl 1 s 22 p 63 s 23 p 6

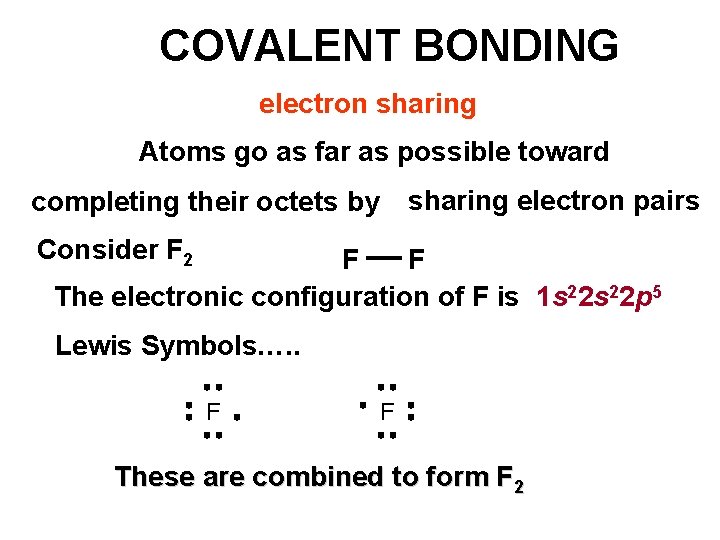
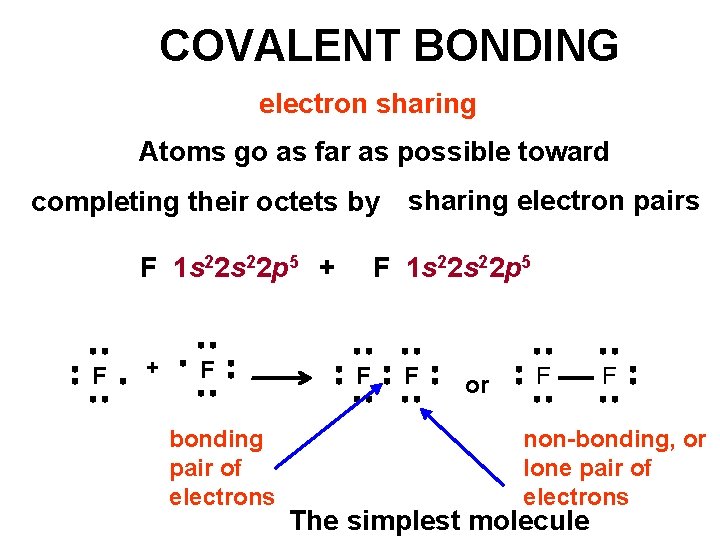
COVALENT BONDING electron sharing Atoms go as far as possible toward sharing electron pairs completing their octets by Consider F 2 F F The electronic configuration of F is 1 s 22 p 5 Lewis Symbols…. . F F These are combined to form F 2

COVALENT BONDING electron sharing Atoms go as far as possible toward completing their octets by F 1 s 22 p 5 + F bonding pair of electrons sharing electron pairs F 1 s 22 p 5 F F or F F non-bonding, or lone pair of electrons The simplest molecule

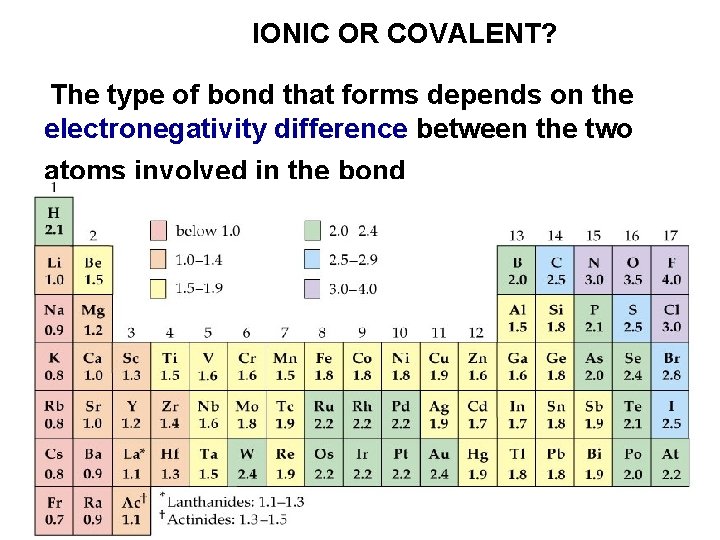
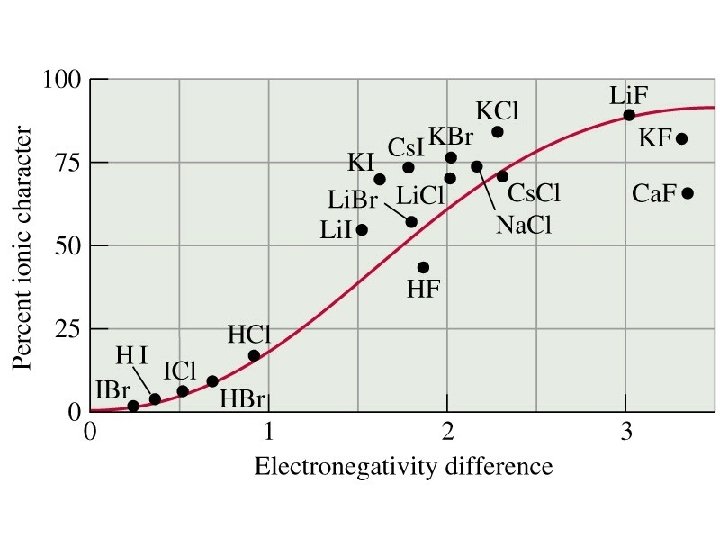
IONIC OR COVALENT? The type of bond that forms depends on the electronegativity difference between the two atoms involved in the bond

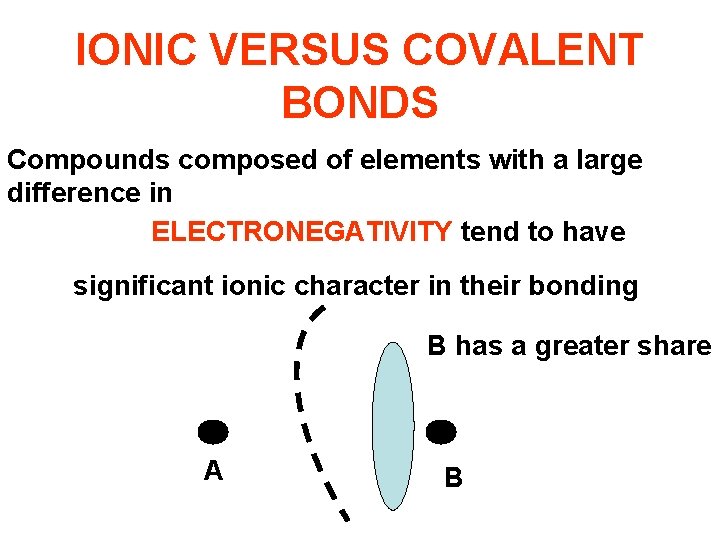
IONIC VERSUS COVALENT BONDS Compounds composed of elements with a large difference in ELECTRONEGATIVITY tend to have significant ionic character in their bonding B has a greater share A B

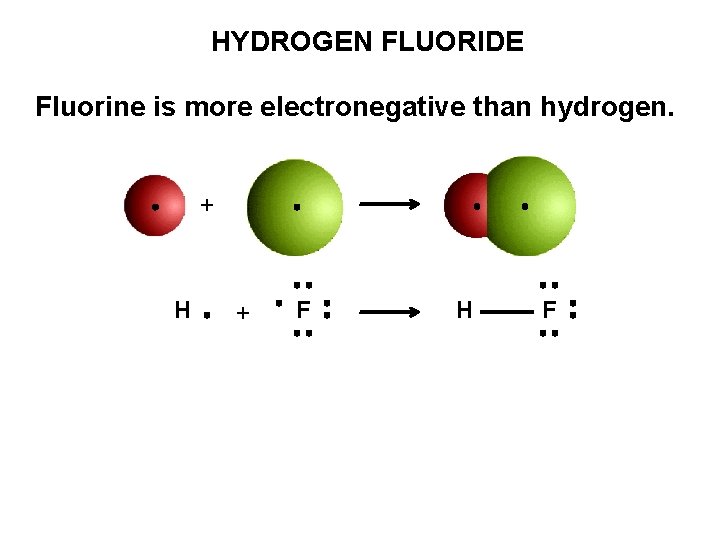
HYDROGEN FLUORIDE Fluorine is more electronegative than hydrogen. + H + F H F


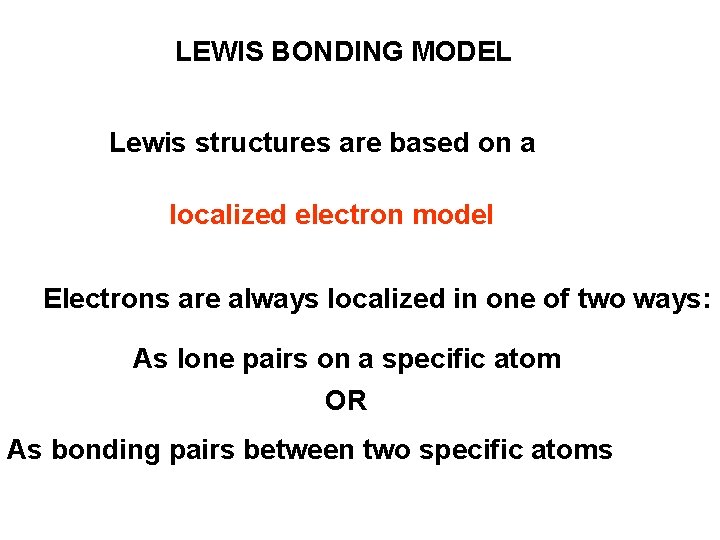
LEWIS BONDING MODEL Lewis structures are based on a localized electron model Electrons are always localized in one of two ways: As lone pairs on a specific atom OR As bonding pairs between two specific atoms

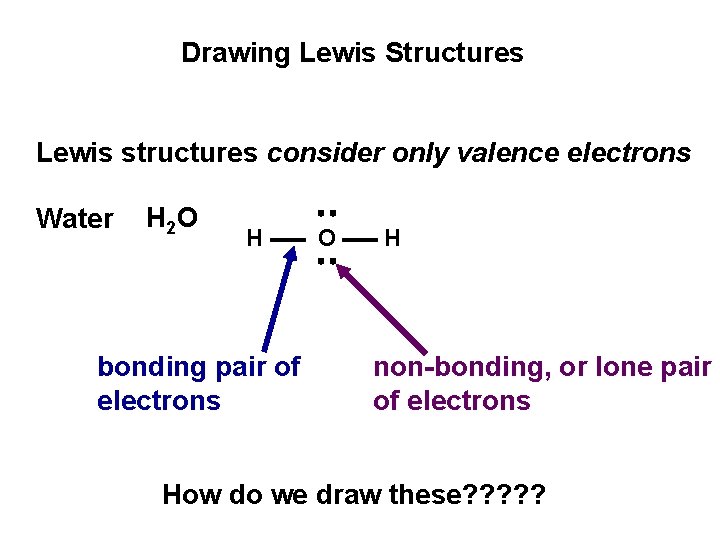
Drawing Lewis Structures Lewis structures consider only valence electrons Water H 2 O H bonding pair of electrons O H non-bonding, or lone pair of electrons How do we draw these? ? ?


Building Lewis (ELECTRON DOT) Structures of Molecules. HCN as an example. . . Step 1. Count the total number of valence electrons H has 1 Total of 10 N has 5 Step 2. Place one e- pair between each BONDED atom C has 4 H C N We have 6 e- left All atoms must have an octet or duet Step 3. Add electrons to terminal atoms first to get an octet or duet.

Building Lewis (ELECTRON DOT) Structures of Molecules. Step 3. Add remaining electrons to terminal atoms first Add 6 electrons in pairs to give the N an octet. H C N Step 4. Add any electrons left over to central atom We have none left! Step 5. Check for an acceptable Lewis Structure Do all atoms have an octet? IN THIS CASE

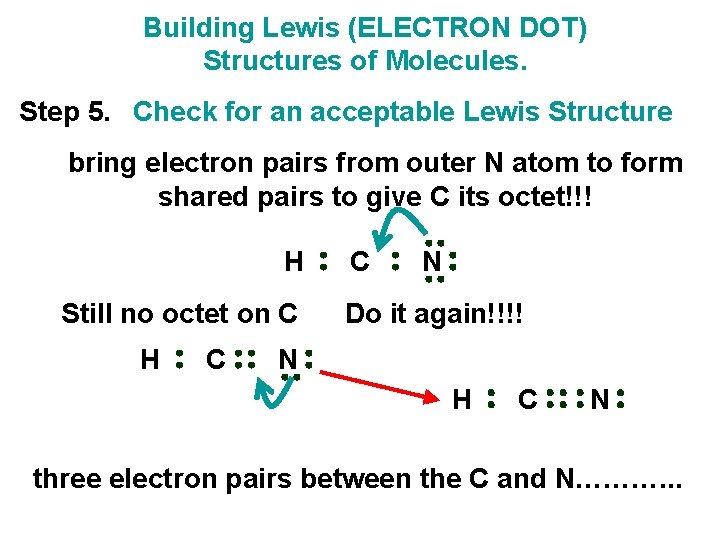
Building Lewis (ELECTRON DOT) Structures of Molecules. Step 5. Check for an acceptable Lewis Structure bring electron pairs from outer N atom to form shared pairs to give C its octet!!! H Still no octet on C H C C N Do it again!!!! N H C N three electron pairs between the C and N………. . .

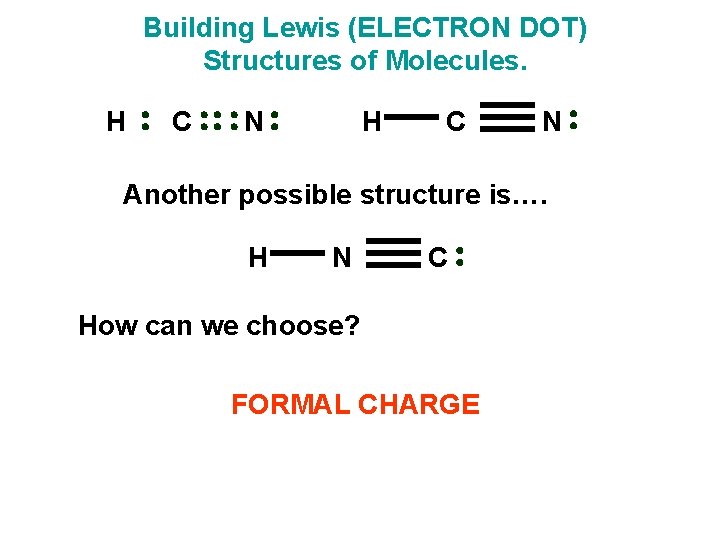
Building Lewis (ELECTRON DOT) Structures of Molecules. H C N Another possible structure is…. H N C How can we choose? FORMAL CHARGE

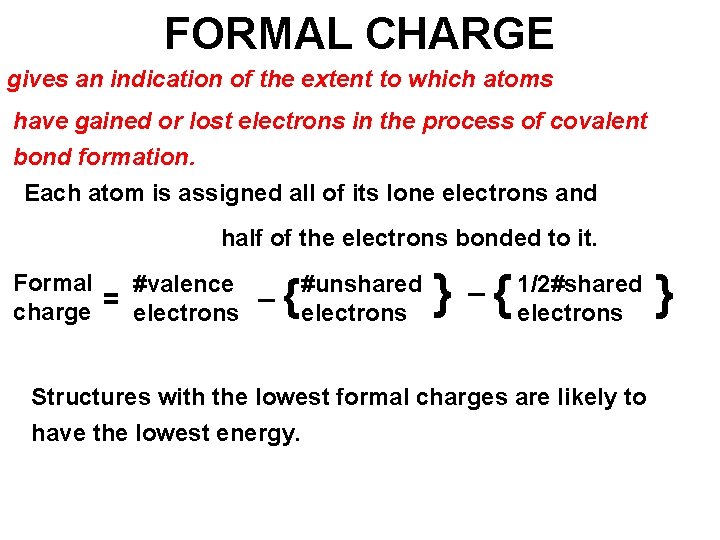
FORMAL CHARGE gives an indication of the extent to which atoms have gained or lost electrons in the process of covalent bond formation. Each atom is assigned all of its lone electrons and half of the electrons bonded to it. Formal #valence _ = charge electrons { #unshared electrons _ } { 1/2#shared electrons Structures with the lowest formal charges are likely to have the lowest energy. }

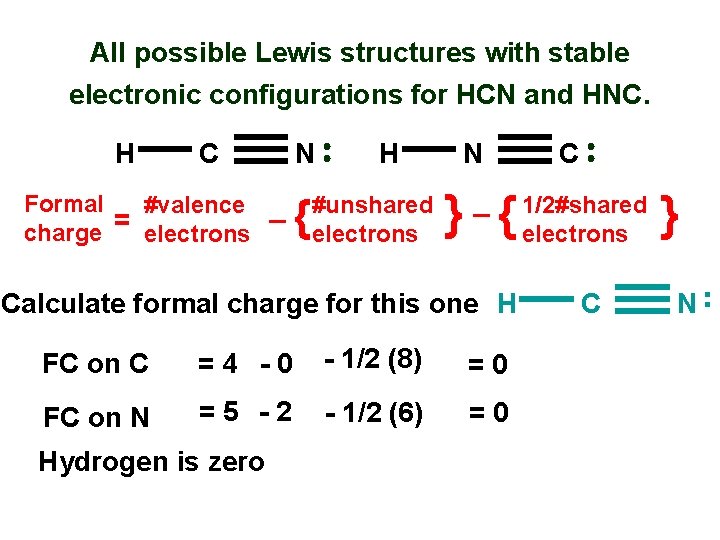
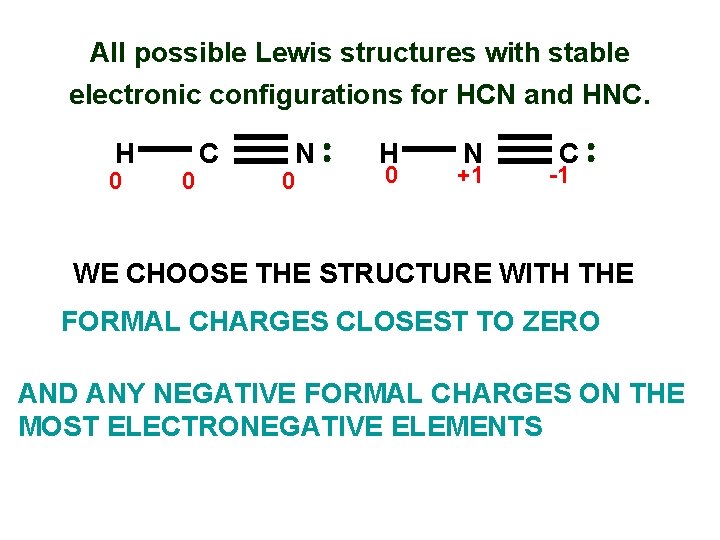
All possible Lewis structures with stable electronic configurations for HCN and HNC. H C Formal #valence _ charge = electrons N { H #unshared electrons N _ } { FC on C =4 -0 - 1/2 (8) =0 FC on N =5 -2 - 1/2 (6) =0 Hydrogen is zero 1/2#shared electrons C } N . . Calculate formal charge for this one H C

All possible Lewis structures with stable electronic configurations for HCN and HNC. H 0 0 C N 0 H 0 N +1 C -1 WE CHOOSE THE STRUCTURE WITH THE FORMAL CHARGES CLOSEST TO ZERO AND ANY NEGATIVE FORMAL CHARGES ON THE MOST ELECTRONEGATIVE ELEMENTS

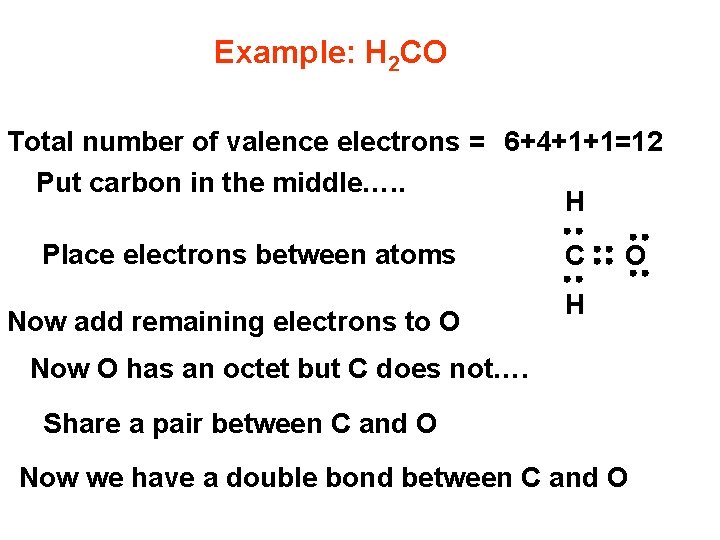
Example: H 2 CO Total number of valence electrons = 6+4+1+1=12 Put carbon in the middle…. . H Place electrons between atoms Now add remaining electrons to O C O H Now O has an octet but C does not…. Share a pair between C and O Now we have a double bond between C and O

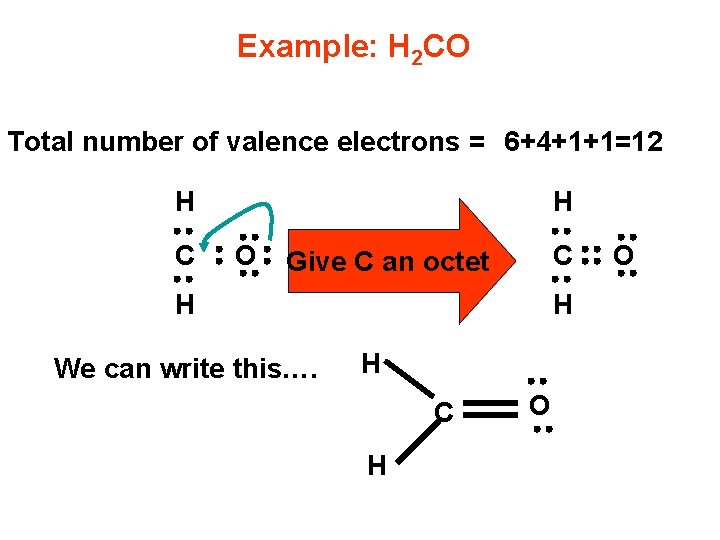
Example: H 2 CO Total number of valence electrons = 6+4+1+1=12 H C H O C Give C an octet H We can write this…. H H C H O O

Let’s look at the nitrate anion NO 3 - Count up valence electrons N has five 1 s 22 p 3 O has six 1 s 22 p 4 Plus one extra for negative charge Valence electrons = 5 + 3 x 6 + 1 = 24

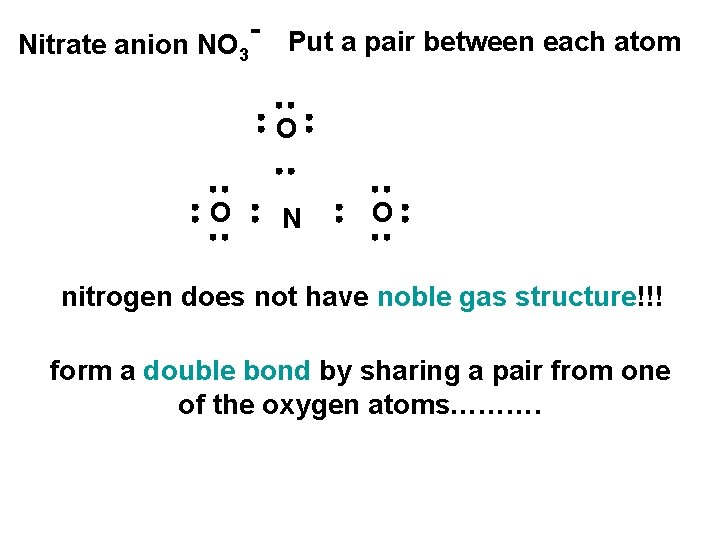
Nitrate anion NO 3 Put a pair between each atom O O N O nitrogen does not have noble gas structure!!! form a double bond by sharing a pair from one of the oxygen atoms……….

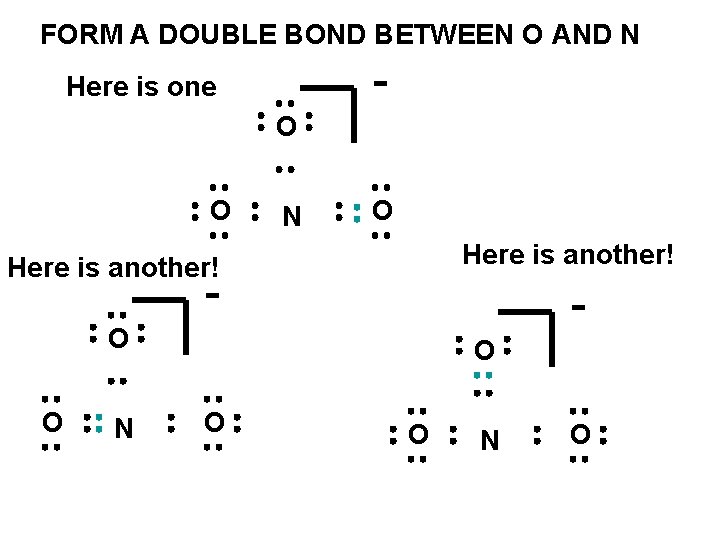
FORM A DOUBLE BOND BETWEEN O AND N - Here is one O O N O Here is another! - - O O N O

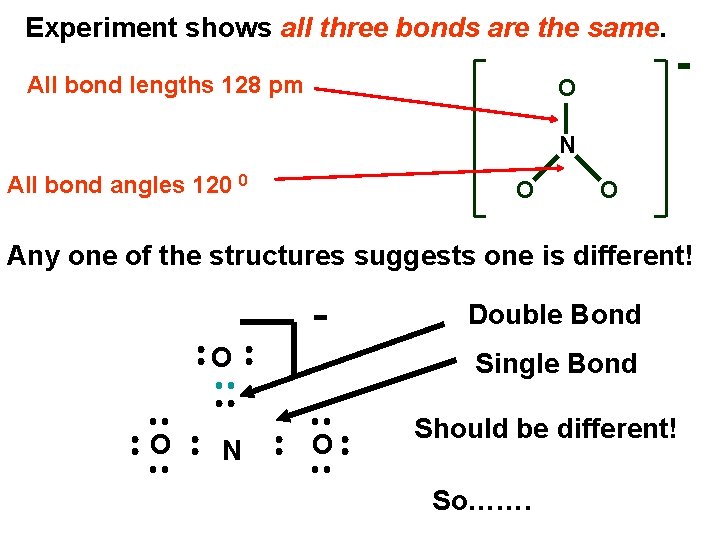
Experiment shows all three bonds are the same. All bond lengths 128 pm O N All bond angles 120 0 O O Any one of the structures suggests one is different! O O N Double Bond Single Bond O Should be different! So…….

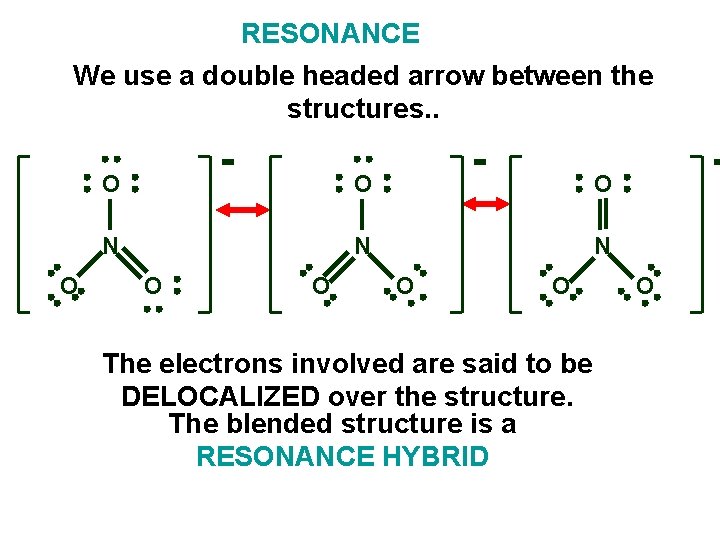
RESONANCE We use a double headed arrow between the structures. . O O N N N O O The electrons involved are said to be DELOCALIZED over the structure. The blended structure is a RESONANCE HYBRID O

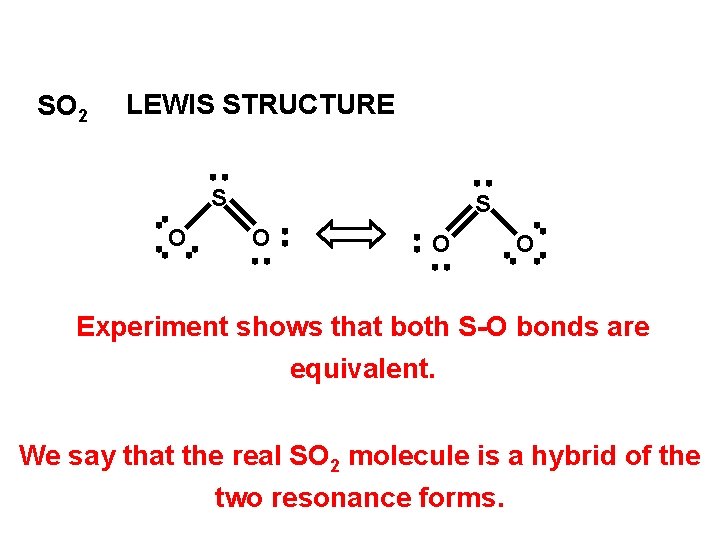
SO 2 LEWIS STRUCTURE S O O O Experiment shows that both S-O bonds are equivalent. We say that the real SO 2 molecule is a hybrid of the two resonance forms.

EXCEPTIONS TO THE OCTET RULE……. Molecules with more than 8 electrons around central atom. Molecules with less than an octet around central atom Molecules with unpaired electrons. Lets do SF 6……. .

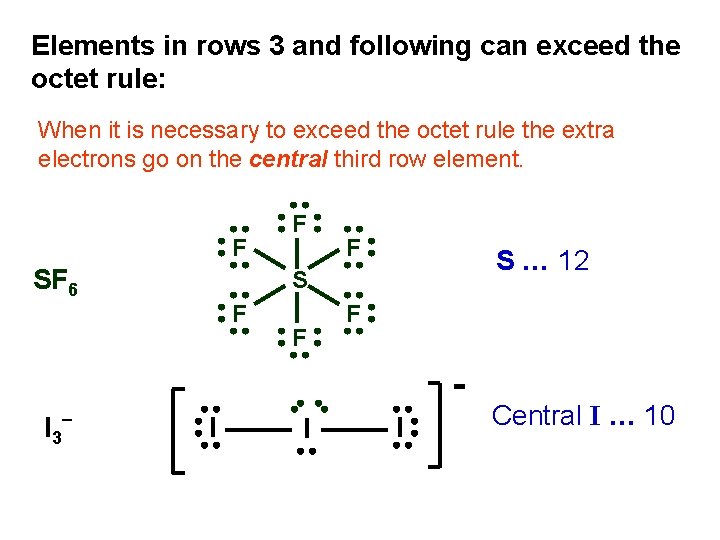
Elements in rows 3 and following can exceed the octet rule: When it is necessary to exceed the octet rule the extra electrons go on the central third row element. F SF 6 F S … 12 S F I 3 - F F F I I I Central I … 10

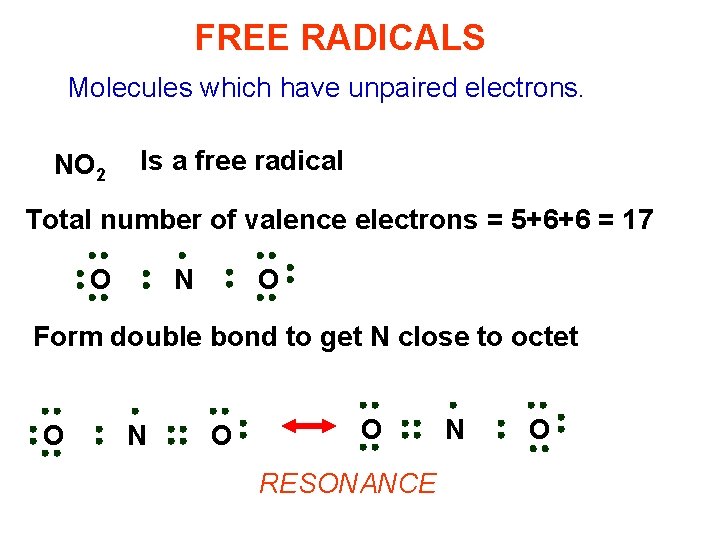
FREE RADICALS Molecules which have unpaired electrons. NO 2 Is a free radical Total number of valence electrons = 5+6+6 = 17 O N O Form double bond to get N close to octet O N O O RESONANCE N O