13 2 Chemical Formulas Octet Rule n Atoms









- Slides: 16

13. 2 Chemical Formulas

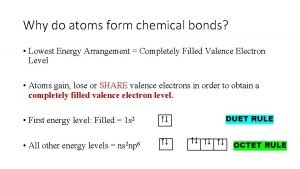
Octet Rule n Atoms form bonds to have 8 valence electrons – Full outer energy level – Makes atoms stable Ne

Ions n n Ions form when atoms gain or lose electrons to satisfy the octet rule Get a charge – Gain = negative charge (more e- than p+) – Lose = positive charge (more p+ than e-) n Easier to gain/lose less electrons!

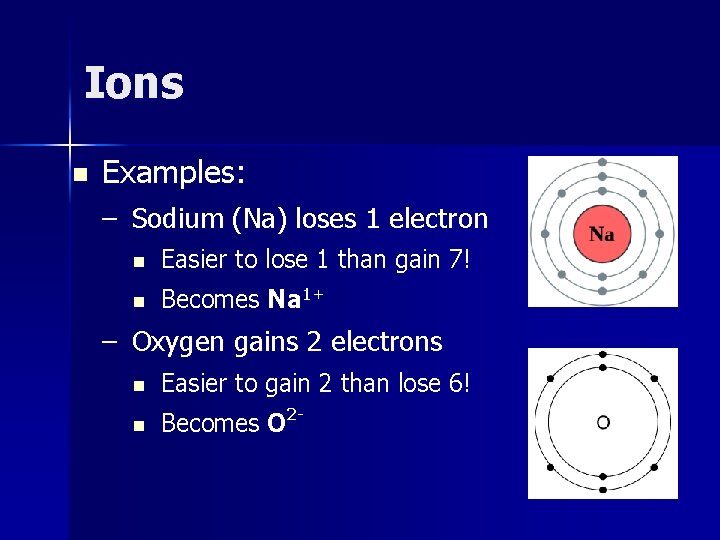
Ions n Examples: – Sodium (Na) loses 1 electron n Easier to lose 1 than gain 7! n Becomes Na 1+ – Oxygen gains 2 electrons n Easier to gain 2 than lose 6! n Becomes O 2 -

Check in! n How many valence electrons does Magnesium have? – Will it gain/lose and how many? – What does a Magnesium ion look like? n How many valence electrons does Phosphorus have? – Will it gain/lose and how many? – What does a Phosphorus ion look like?

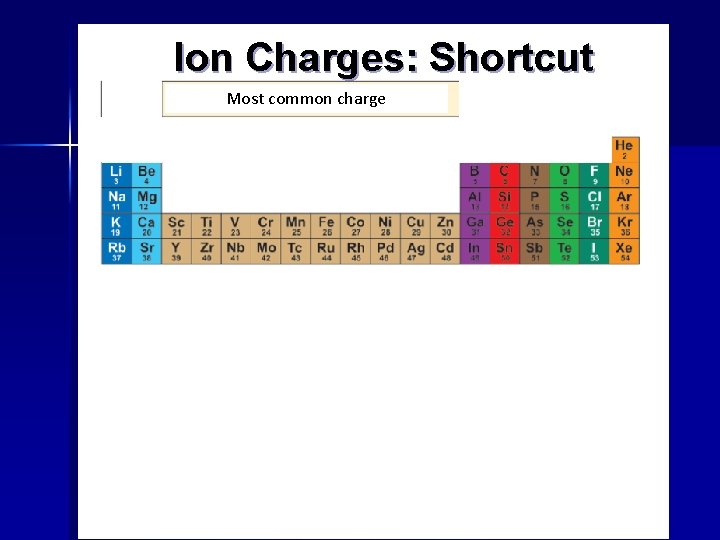
Ion Charges: Shortcut Most common charge Lose one electron Lose two electrons Gain one electron

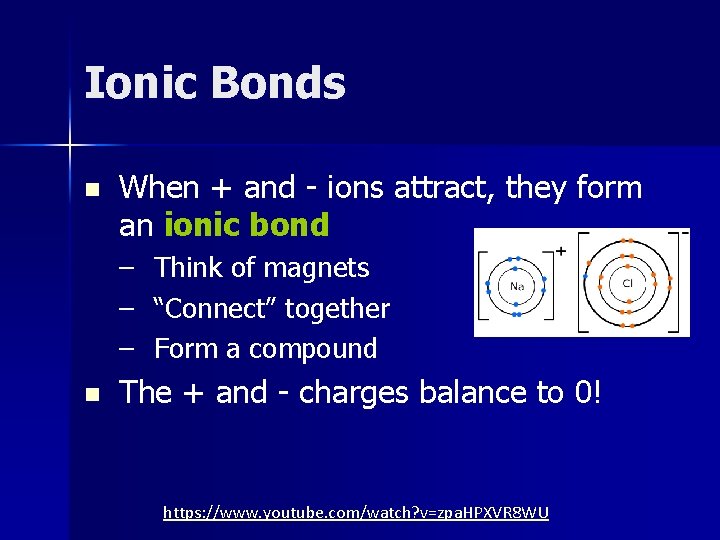
Ionic Bonds n When + and - ions attract, they form an ionic bond – Think of magnets – “Connect” together – Form a compound n The + and - charges balance to 0! https: //www. youtube. com/watch? v=zpa. HPXVR 8 WU

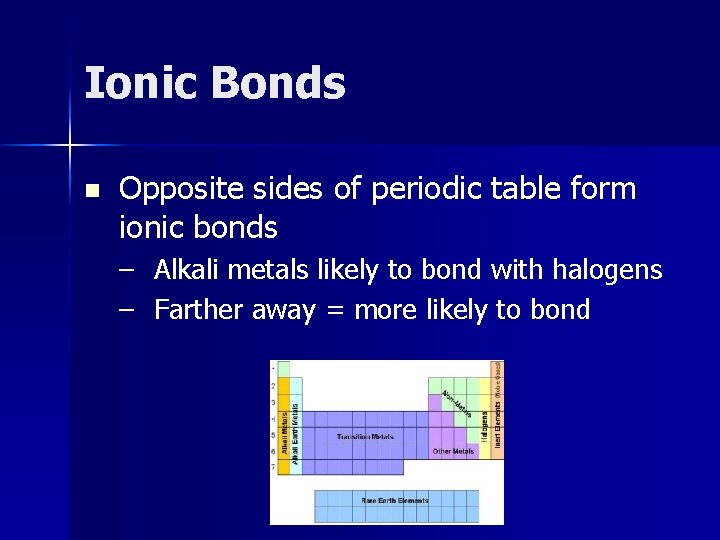
Ionic Bonds n Opposite sides of periodic table form ionic bonds – Alkali metals likely to bond with halogens – Farther away = more likely to bond

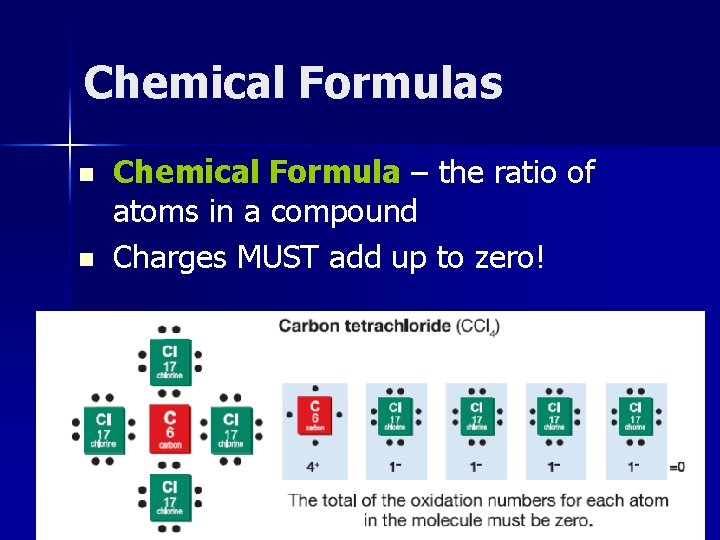
Chemical Formulas n n Chemical Formula – the ratio of atoms in a compound Charges MUST add up to zero!

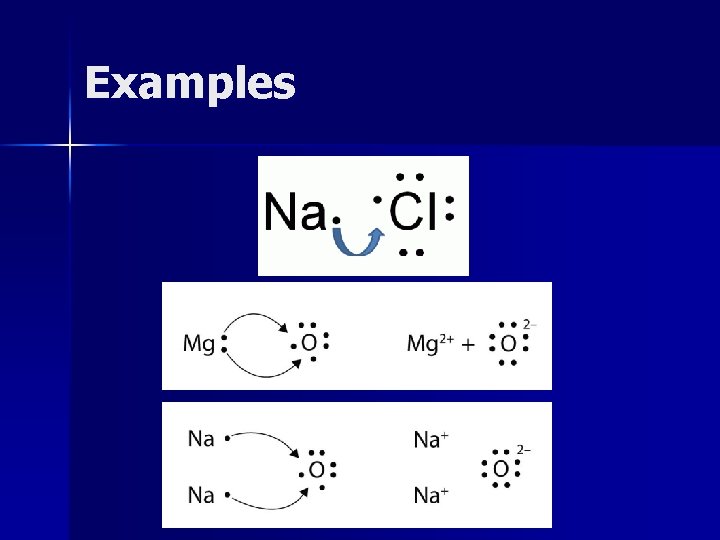
Showing Ionic Bonds n To write: – 1 - Draw Lewis dot structures – 2 - Determine which element loses electrons – Hint: which has less valence electrons? – 3 - Draw arrow(s) to show electron transfer – 4 - Add atoms as needed

Examples

Check In n Draw the transfer of electrons for each pair of atoms: – Na and Br (as a class) – Mg and F (as a class) – Li and O (you try)

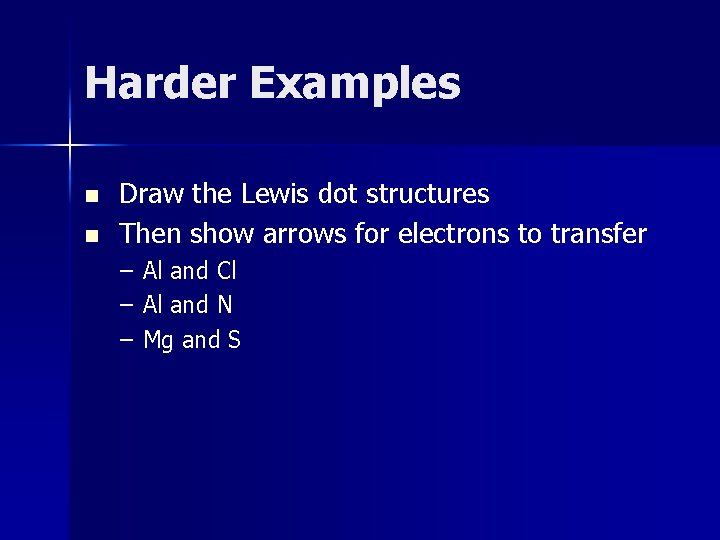
Harder Examples n n Draw the Lewis dot structures Then show arrows for electrons to transfer – – – Al and Cl Al and N Mg and S

Writing Chemical Formulas n n 1. Show the transfer of valence electrons 2. Count up atoms of each element 3. Write metal first, nonmetal second 4. Add subscripts to indicate # of atoms Example: Mg and F

Work Time n Work on your homework packet for the rest of the hour!

Writing Chemical Formulas – EXIT SLIP Aluminum and Chlorine combine to form a compound a. How many valence electrons does each element have? b. What are the Lewis dot diagrams of each atom? c. What are the charges of each element? d. What is the chemical formula of this compound?
 Counting atoms
Counting atoms Why do atoms form chemical bonds? *
Why do atoms form chemical bonds? * H2bf lewis structure
H2bf lewis structure What atoms can have an expanded octet
What atoms can have an expanded octet Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chemical formulas and chemical compounds chapter 7
Chemical formulas and chemical compounds chapter 7 At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity The octet rule states that
The octet rule states that What is octet rule
What is octet rule No+ vsepr
No+ vsepr Septa prefix
Septa prefix Whats ionic bond
Whats ionic bond Octet rule exceptions
Octet rule exceptions The octet rule states that
The octet rule states that The octet rule states that
The octet rule states that Mg octet rule
Mg octet rule Aluminum chloride charge
Aluminum chloride charge