Results of the Repatha Outcomes Study 2 PSCK








- Slides: 26

Results of the Repatha® Outcomes Study 2 PSCK 9 = Proprotein convertase subtilisin/kexin type 9 1. Repatha® (evolocumab) Summary of Product Characteristics 2. Sabatine MS, et al. N Eng J Med. 2017; 376: 1713 UKIE-P-145 -0318 -062527(6) April 2020 22 Repatha® (evolocumab) prescribing information available on final slide

Cardiovascular Disease Represents a Major Burden in the UK

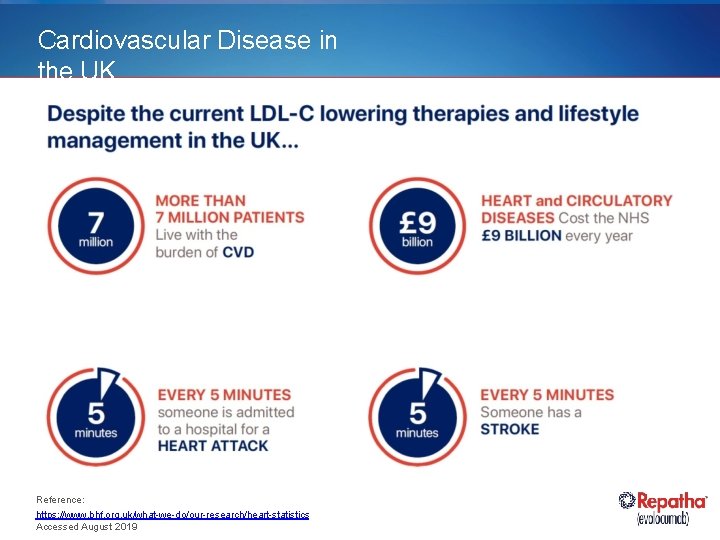
Cardiovascular Disease in the UK Reference: https: //www. bhf. org. uk/what-we-do/our-research/heart-statistics Accessed August 2019

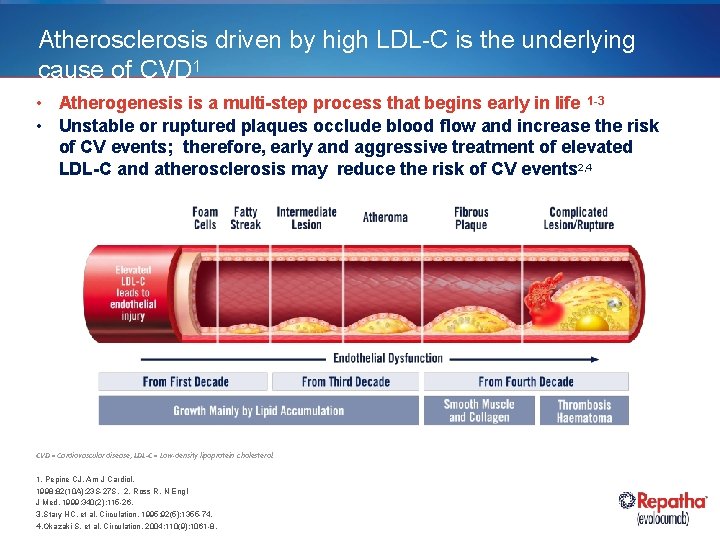
Atherosclerosis driven by high LDL-C is the underlying cause of CVD 1 • Atherogenesis is a multi-step process that begins early in life 1 -3 • Unstable or ruptured plaques occlude blood flow and increase the risk of CV events; therefore, early and aggressive treatment of elevated LDL-C and atherosclerosis may reduce the risk of CV events 2, 4 CVD = Cardiovascular disease; LDL-C = Low-density lipoprotein cholesterol. 1. Pepine CJ. Am J Cardiol. 1998; 82(10 A): 23 S-27 S. 2. Ross R. N Engl J Med. 1999; 340(2): 115 -26. 3. Stary HC, et al. Circulation. 1995; 92(5): 1355 -74. 4. Okazaki S, et al. Circulation. 2004; 110(9): 1061 -8.

There Are Many Patients Who Need to Control Their LDL-C Levels Beyond Current Therapy LDL-C = Low-density lipoprotein cholesterol.

For some patients, statins alone won’t be enough to lower their LDL-C to target 1. Cholesterol Treatment Trialists’ (CTT) Collaborators. Lancet. 2010; 376: 1670– 81 2. Danese MD et al. Curr Med Res Opin. 2018; 34: 1441 -47 3. Data on file 4. Mach F et al. , Eur Heart J. 2019 ehz 455,

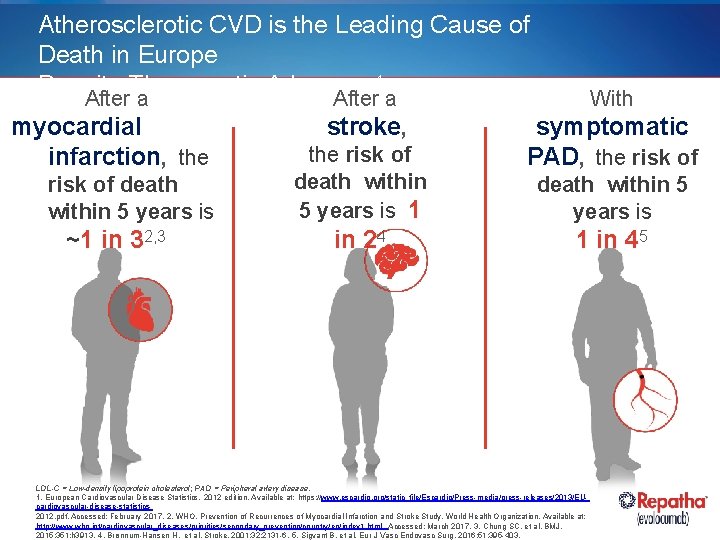
Atherosclerotic CVD is the Leading Cause of Death in Europe Despite Therapeutic Advances 1 After a myocardial infarction, the risk of death within 5 years is ~1 in 32, 3 After a With stroke, symptomatic PAD, the risk of death within 5 years is 1 in 24 death within 5 years is 1 in 45 LDL-C = Low-density lipoprotein cholesterol; PAD = Peripheral artery disease. 1. European Cardiovascular Disease Statistics. 2012 edition. Available at: https: //www. escardio. org/static_file/Escardio/Press-media/press-releases/2013/EUcardiovascular-disease-statistics 2012. pdf. Accessed: February 2017. 2. WHO. Prevention of Recurrences of Myocardial Infarction and Stroke Study. World Health Organization. Available at: http: //www. who. int/cardiovascular_diseases/priorities/secondary_prevention/country/en/index 1. html. Accessed: March 2017. 3. Chung SC, et al. BMJ. 2015; 351: h 3913. 4. Brønnum-Hansen H, et al. Stroke. 2001; 32: 2131 -6. 5. Sigvant B, et al. Eur J Vasc Endovasc Surg. 2016; 51: 395 -403.

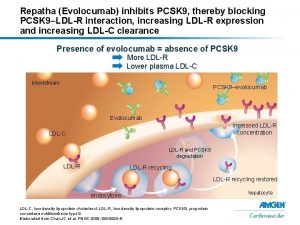
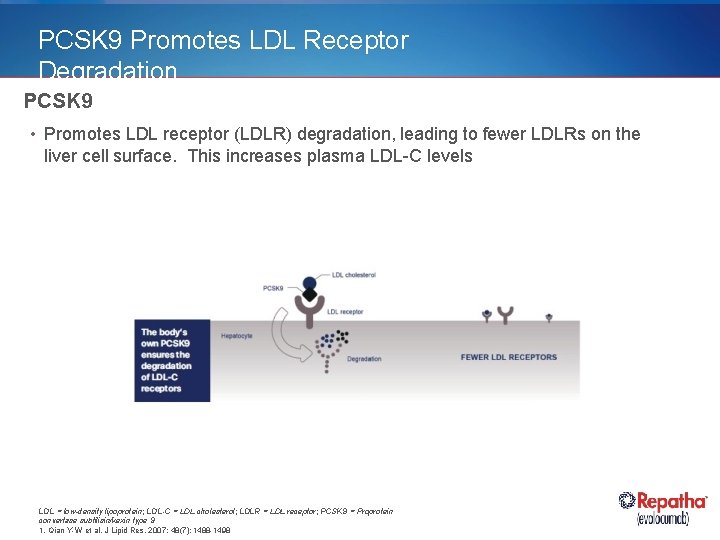
PCSK 9 Promotes LDL Receptor Degradation PCSK 9 • Promotes LDL receptor (LDLR) degradation, leading to fewer LDLRs on the liver cell surface. This increases plasma LDL-C levels LDL = low-density lipoprotein; LDL-C = LDL cholesterol; LDLR = LDL receptor; PCSK 9 = Proprotein convertase subtilisin/kexin type 9 1. Qian Y-W et al. J Lipid Res. 2007; 48(7): 1488 -1498

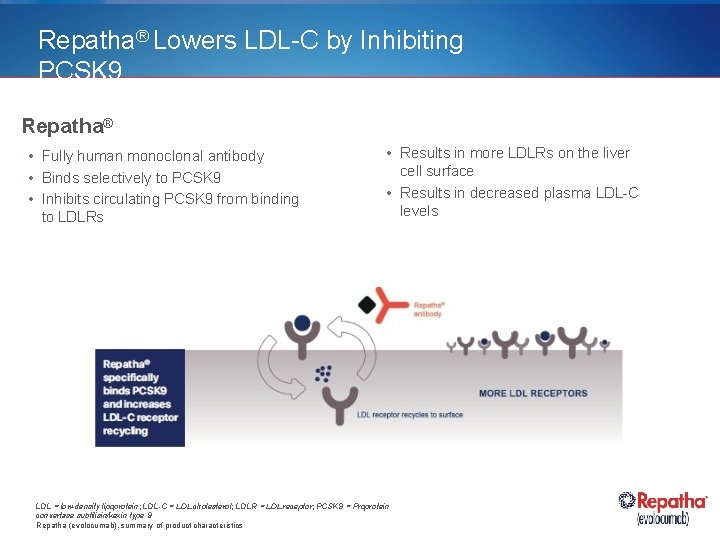
Repatha® Lowers LDL-C by Inhibiting PCSK 9 Repatha® • Fully human monoclonal antibody • Binds selectively to PCSK 9 • Inhibits circulating PCSK 9 from binding to LDLRs • Results in more LDLRs on the liver cell surface • Results in decreased plasma LDL-C levels LDL = low-density lipoprotein; LDL-C = LDL cholesterol; LDLR = LDL receptor; PCSK 9 = Proprotein convertase subtilisin/kexin type 9 Repatha (evolocumab), summary of product characteristics

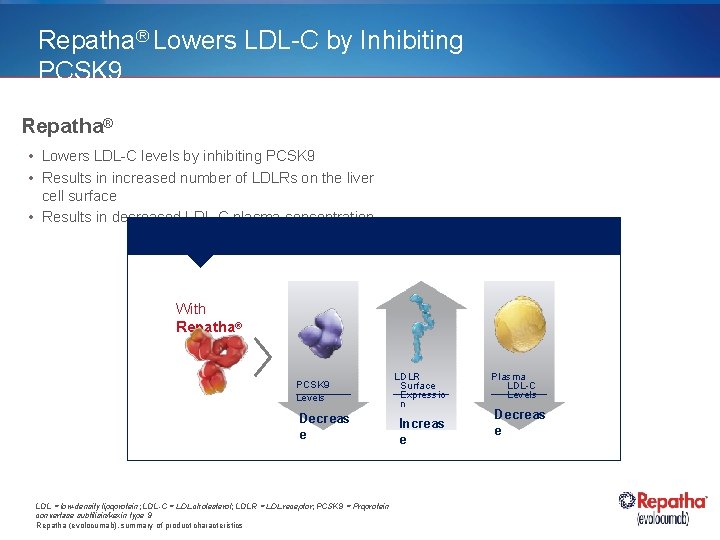
Repatha® Lowers LDL-C by Inhibiting PCSK 9 Repatha® • Lowers LDL-C levels by inhibiting PCSK 9 • Results in increased number of LDLRs on the liver cell surface • Results in decreased LDL-C plasma concentration With Repatha® PCSK 9 Levels Decreas e LDL = low-density lipoprotein; LDL-C = LDL cholesterol; LDLR = LDL receptor; PCSK 9 = Proprotein convertase subtilisin/kexin type 9 Repatha (evolocumab), summary of product characteristics LDLR Surface Expressio n Increas e Plasma LDL-C Levels Decreas e

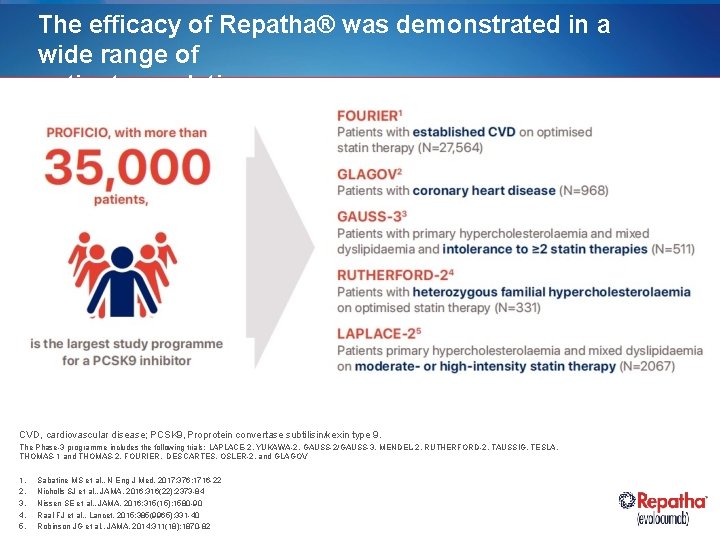
The efficacy of Repatha® was demonstrated in a wide range of patient populations CVD, cardiovascular disease; PCSK 9, Proprotein convertase subtilisin/kexin type 9. The Phase-3 programme includes the following trials: LAPLACE-2, YUKAWA-2, GAUSS-2/GAUSS-3, MENDEL-2, RUTHERFORD-2, TAUSSIG, TESLA, THOMAS-1 and THOMAS-2, FOURIER, DESCARTES, OSLER-2, and GLAGOV 1. 2. 3. 4. 5. Sabatine MS et al. , N Eng J Med. 2017; 376: 1716 -22 Nicholls SJ et al. , JAMA. 2016; 316(22): 2373 -84 Nissen SE et al. , JAMA. 2016; 315(15): 1580 -90 Raal FJ et al. , Lancet. 2015; 385(9965): 331 -40 Robinson JG et al. , JAMA. 2014; 311(18): 1870 -82

Repatha®: Therapeutic Indications 1 Hypercholesterolaemia and mixed dyslipidaemia Repatha is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet: • in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin or, • alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. Homozygous familial hypercholesterolaemia Repatha is indicated in adults and adolescents aged 12 years and over with homozygous familial hypercholesterolaemia in combination with other lipid-lowering therapies. Established atherosclerotic cardiovascular disease Repatha is indicated in adults with established atherosclerotic cardiovascular disease (myocardial infarction, stroke or peripheral arterial disease) to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors: • in combination with the maximum tolerated dose of a statin with or without other lipid-lowering therapies or, • alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. For study results with respect to effects on LDL-C, cardiovascular events and populations studied see section 5. 1 of the SPC LDL-C = Low-density lipoprotein cholesterol. 1. Repatha® (evolocumab) Summary of Product Characteristics.

Repatha® Outcomes Study – FOURIER

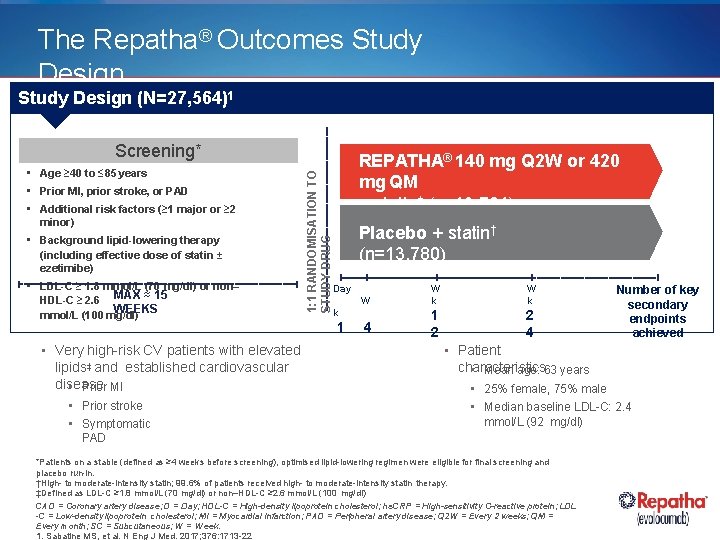
The Repatha® Outcomes Study Design (N=27, 564)1 • Age ≥ 40 to ≤ 85 years • Prior MI, prior stroke, or PAD • Additional risk factors (≥ 1 major or ≥ 2 minor) • Background lipid-lowering therapy (including effective dose of statin ± ezetimibe) • LDL-C ≥ 1. 8 mmol/L (70 mg/dl) or non– HDL-C ≥ 2. 6 MAX ≈ 15 WEEKS mmol/L (100 mg/dl) • Very high-risk CV patients with elevated lipids‡ and established cardiovascular disease • Prior MI • Prior stroke • Symptomatic 1: 1 RANDOMISATION TO STUDY DRUG Screening* REPATHA® 140 mg Q 2 W or 420 mg QM + statin† (n=13, 784) Placebo + statin† (n=13, 780) Day W W k 4 1 2 2 4 k 1 Number of key secondary endpoints achieved • Patient characteristics • Mean age: 63 years • 25% female, 75% male • Median baseline LDL-C: 2. 4 mmol/L (92 mg/dl) PAD *Patients on a stable (defined as ≥ 4 weeks before screening), optimised lipid-lowering regimen were eligible for final screening and placebo run-in. †High- to moderate-intensity statin; 99. 6% of patients received high- to moderate-intensity statin therapy. ‡Defined as LDL-C ≥ 1. 8 mmol/L (70 mg/dl) or non–HDL-C ≥ 2. 6 mmol/L (100 mg/dl) CAD = Coronary artery disease; D = Day; HDL-C = High-density lipoprotein cholesterol; hs. CRP = High-sensitivity C-reactive protein; LDL -C = Low-density lipoprotein cholesterol; MI = Myocardial infarction; PAD = Peripheral artery disease; Q 2 W = Every 2 weeks; QM = Every month; SC = Subcutaneous; W = Week. 1. Sabatine MS, et al. N Eng J Med. 2017; 376: 1713 -22

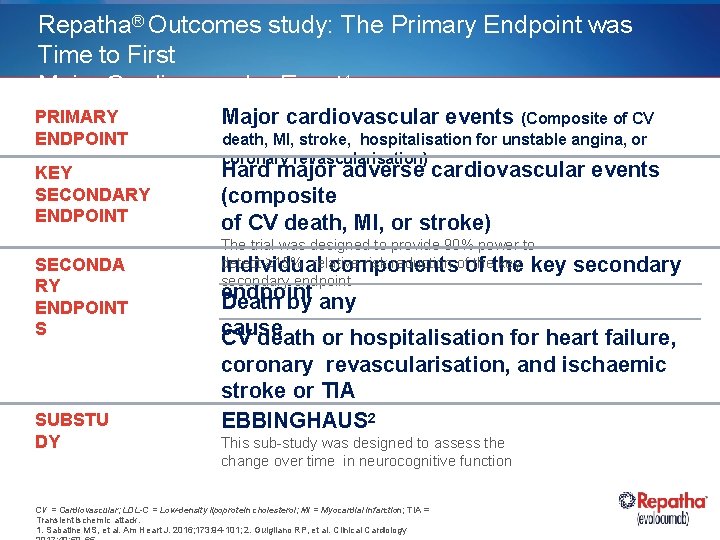
Repatha® Outcomes study: The Primary Endpoint was Time to First Major Cardiovascular Event 1 PRIMARY ENDPOINT Major cardiovascular events (Composite of CV KEY SECONDARY ENDPOINT Hard major adverse cardiovascular events (composite of CV death, MI, or stroke) SECONDA RY ENDPOINT S SUBSTU DY death, MI, stroke, hospitalisation for unstable angina, or coronary revascularisation) The trial was designed to provide 90% power to detect ≥ 15% relative risk reduction ofof thethe key Individual components secondary endpoint Death by any cause CV death or hospitalisation for heart failure, coronary revascularisation, and ischaemic stroke or TIA EBBINGHAUS 2 This sub-study was designed to assess the change over time in neurocognitive function CV = Cardiovascular; LDL-C = Low-density lipoprotein cholesterol; MI = Myocardial infarction; TIA = Transient ischemic attack. 1. Sabatine MS, et al. Am Heart J. 2016; 173: 94 -101; 2. Guigliano RP, et al. Clinical Cardiology

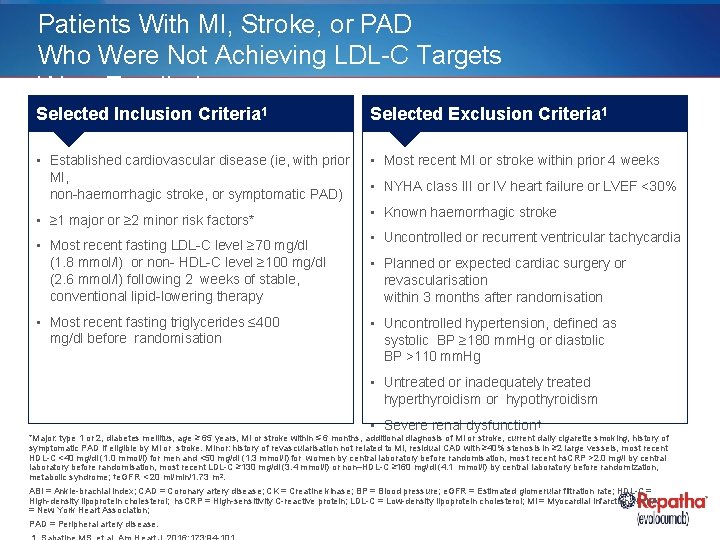
Patients With MI, Stroke, or PAD Who Were Not Achieving LDL-C Targets Were Enrolled Selected Inclusion Criteria 1 Selected Exclusion Criteria 1 • Established cardiovascular disease (ie, with prior MI, non-haemorrhagic stroke, or symptomatic PAD) • Most recent MI or stroke within prior 4 weeks • ≥ 1 major or ≥ 2 minor risk factors* • Most recent fasting LDL-C level ≥ 70 mg/dl (1. 8 mmol/l) or non- HDL-C level ≥ 100 mg/dl (2. 6 mmol/l) following 2 weeks of stable, conventional lipid-lowering therapy • Most recent fasting triglycerides ≤ 400 mg/dl before randomisation • NYHA class III or IV heart failure or LVEF <30% • Known haemorrhagic stroke • Uncontrolled or recurrent ventricular tachycardia • Planned or expected cardiac surgery or revascularisation within 3 months after randomisation • Uncontrolled hypertension, defined as systolic BP ≥ 180 mm. Hg or diastolic BP >110 mm. Hg • Untreated or inadequately treated hyperthyroidism or hypothyroidism • Severe renal dysfunction† *Major: type 1 or 2, diabetes mellitus, age ≥ 65 years, MI or stroke within ≤ 6 months, additional diagnosis of MI or stroke, current daily cigarette smoking, history of symptomatic PAD if eligible by MI or stroke. Minor: history of revascularisation not related to MI, residual CAD with ≥ 40% stenosis in ≥ 2 large vessels, most recent HDL-C <40 mg/dl (1. 0 mmol/l) for men and <50 mg/dl (1. 3 mmol/l) for women by central laboratory before randomisation, most recent hs. CRP >2. 0 mg/l by central laboratory before randomisation, most recent LDL-C ≥ 130 mg/dl (3. 4 mmol/l) or non–HDL-C ≥ 160 mg/dl (4. 1 mmol/l) by central laboratory before randomization, metabolic syndrome; †e. GFR < 20 ml/min/1. 73 m 2. ABI = Ankle-brachial index; CAD = Coronary artery disease; CK = Creatine kinase; BP = Blood pressure; e. GFR = Estimated glomerular filtration rate; HDL-C = High-density lipoprotein cholesterol; hs. CRP = High-sensitivity C-reactive protein; LDL-C = Low-density lipoprotein cholesterol; MI = Myocardial infarction; NYHA = New York Heart Association; PAD = Peripheral artery disease.

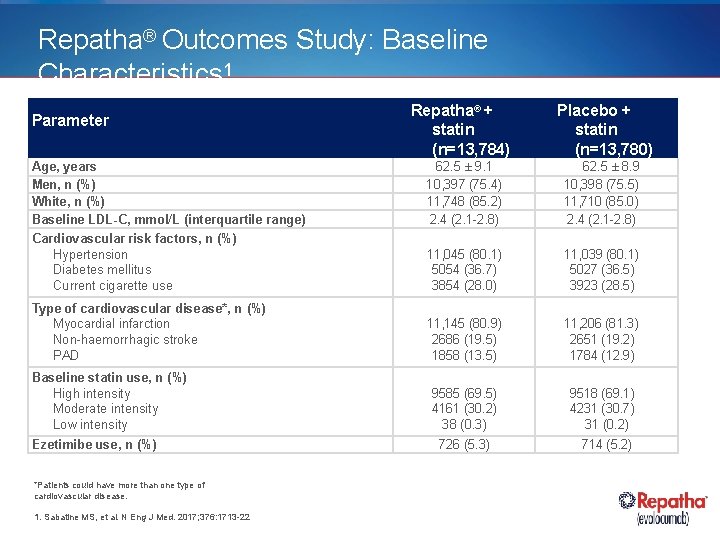
Repatha® Outcomes Study: Baseline Characteristics 1 Parameter Repatha® + statin (n=13, 784) Placebo + statin (n=13, 780) Age, years Men, n (%) White, n (%) Baseline LDL-C, mmol/L (interquartile range) 62. 5 ± 9. 1 10, 397 (75. 4) 11, 748 (85. 2) 2. 4 (2. 1 -2. 8) 62. 5 ± 8. 9 10, 398 (75. 5) 11, 710 (85. 0) 2. 4 (2. 1 -2. 8) Cardiovascular risk factors, n (%) Hypertension Diabetes mellitus Current cigarette use 11, 045 (80. 1) 5054 (36. 7) 3854 (28. 0) 11, 039 (80. 1) 5027 (36. 5) 3923 (28. 5) Type of cardiovascular disease*, n (%) Myocardial infarction Non-haemorrhagic stroke PAD 11, 145 (80. 9) 2686 (19. 5) 1858 (13. 5) 11, 206 (81. 3) 2651 (19. 2) 1784 (12. 9) 9585 (69. 5) 4161 (30. 2) 38 (0. 3) 9518 (69. 1) 4231 (30. 7) 31 (0. 2) Baseline statin use, n (%) High intensity Moderate intensity Low intensity Ezetimibe use, n (%) *Patients could have more than one type of cardiovascular disease. 1. Sabatine MS, et al. N Eng J Med. 2017; 376: 1713 -22 726 (5. 3) 714 (5. 2)

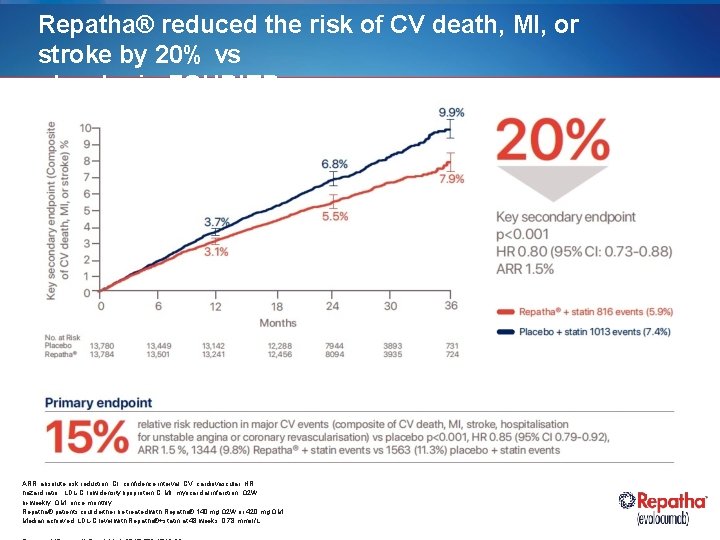
Repatha® reduced the risk of CV death, MI, or stroke by 20% vs placebo in FOURIER ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; HR, hazard ratio; LDL-C, low density lipoprotein C; MI, myocardial infarction; Q 2 W, bi-weekly; QM, once monthly Repatha® patients could either be treated with Repatha® 140 mg Q 2 W or 420 mg QM. Median achieved LDL-C level with Repatha®+statin at 48 weeks: 0. 78 mmol/L

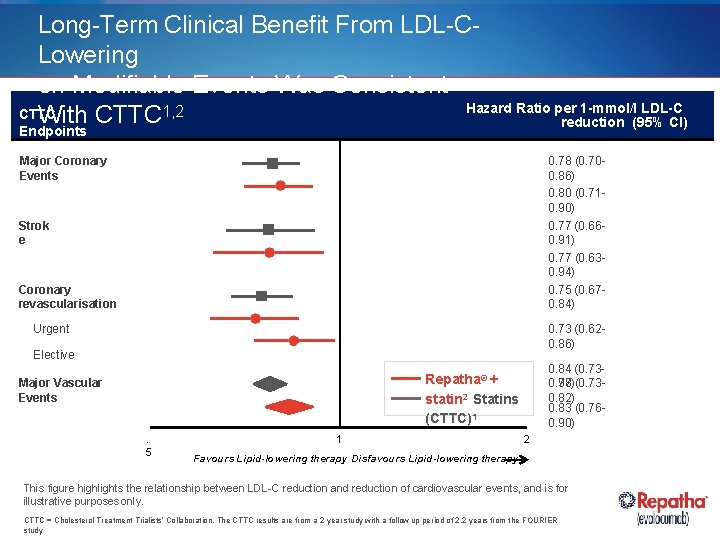
Long-Term Clinical Benefit From LDL-CLowering on Modifiable Events Was Consistent Hazard Ratio per 1 -mmol/l LDL-C CTTC With CTTC 1, 2 reduction (95% CI) Endpoints Major Coronary Events 0. 78 (0. 700. 86) 0. 80 (0. 710. 90) 0. 77 (0. 660. 91) 0. 77 (0. 630. 94) 0. 75 (0. 670. 84) Strok e Coronary revascularisation 0. 73 (0. 620. 86) Urgent Elective 0. 84 (0. 730. 98)(0. 730. 77 0. 82) 0. 83 (0. 760. 90) Repatha® + statin 2 Statins (CTTC)1 Major Vascular Events. 5 1 2 Favours Lipid-lowering therapy Disfavours Lipid-lowering therapy This figure highlights the relationship between LDL-C reduction and reduction of cardiovascular events, and is for illustrative purposes only. CTTC = Cholesterol Treatment Trialists’ Collaboration. The CTTC results are from a 2 year study with a follow up period of 2. 2 years from the FOURIER study

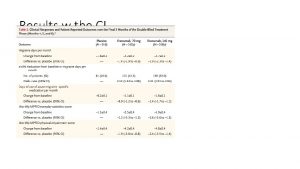
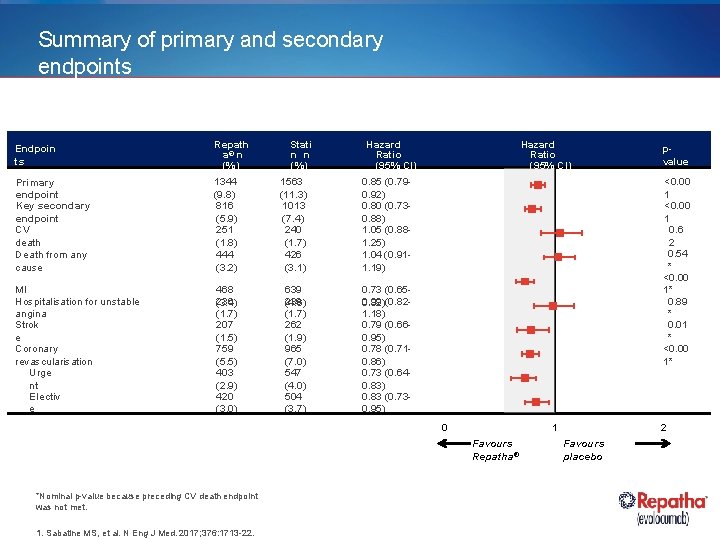
Summary of primary and secondary endpoints Endpoin ts Repath a® n (%) Stati n n (%) Hazard Ratio (95% CI) Primary endpoint Key secondary endpoint CV death Death from any cause 1344 (9. 8) 816 (5. 9) 251 (1. 8) 444 (3. 2) 1563 (11. 3) 1013 (7. 4) 240 (1. 7) 426 (3. 1) 0. 85 (0. 790. 92) 0. 80 (0. 730. 88) 1. 05 (0. 881. 25) 1. 04 (0. 911. 19) MI Hospitalisation for unstable angina Strok e Coronary revascularisation Urge nt Electiv e 468 236 (3. 4) (1. 7) 207 (1. 5) 759 (5. 5) 403 (2. 9) 420 (3. 0) 639 239 (4. 6) (1. 7) 262 (1. 9) 965 (7. 0) 547 (4. 0) 504 (3. 7) 0. 73 (0. 650. 99 0. 82)(0. 821. 18) 0. 79 (0. 660. 95) 0. 78 (0. 710. 86) 0. 73 (0. 640. 83) 0. 83 (0. 730. 95) Hazard Ratio (95% CI) <0. 00 1 0. 6 2 0. 54 * <0. 00 1* 0. 89 * 0. 01 * <0. 00 1* 0 1 Favours Repatha® *Nominal p-value because preceding CV death endpoint was not met. 1. Sabatine MS, et al. N Eng J Med. 2017; 376: 1713 -22. pvalue 2 Favours placebo

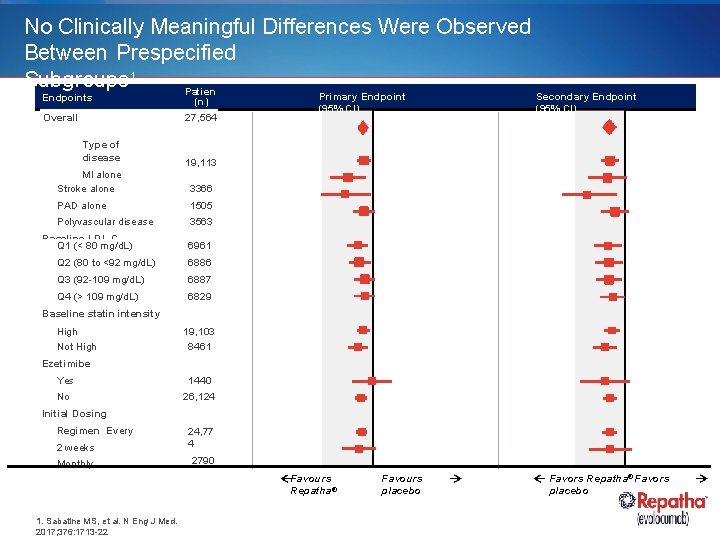
No Clinically Meaningful Differences Were Observed Between Prespecified Subgroups 1 Patien Endpoints Overall Type of disease ts (n) 27, 564 Secondary Endpoint (95% CI) 19, 113 MI alone Stroke alone 3366 PAD alone 1505 Polyvascular disease 3563 Baseline LDL-C Q 1 (< 80 mg/d. L) Primary Endpoint (95% CI) 6961 Q 2 (80 to <92 mg/d. L) 6886 Q 3 (92 -109 mg/d. L) 6887 Q 4 (> 109 mg/d. L) 6829 Baseline statin intensity High Not High 19, 103 8461 Ezetimibe Yes 1440 No 26, 124 Initial Dosing Regimen Every 2 weeks 24, 77 4 Monthly 2790 Favours Repatha® 1. Sabatine MS, et al. N Eng J Med. 2017; 376: 1713 -22 Favours placebo Favors Repatha® Favors placebo

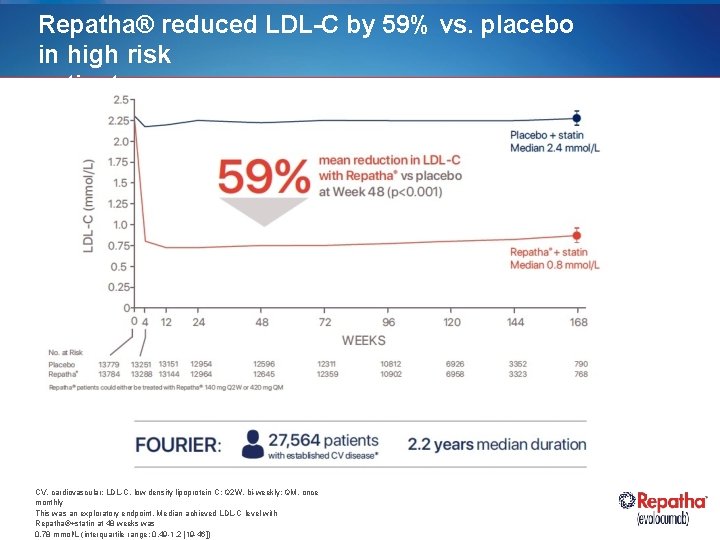
Repatha® reduced LDL-C by 59% vs. placebo in high risk patients CV, cardiovascular; LDL-C, low density lipoprotein C; Q 2 W, bi-weekly; QM, once monthly This was an exploratory endpoint. Median achieved LDL-C level with Repatha®+statin at 48 weeks was 0. 78 mmol/L (interquartile range: 0. 49 -1. 2 [19 -46])

Give patients who are at risk of having another CV event a chance. Start Repatha® CV = Cardiovascular, He. FH = heterozygous familial hypercholesterolaemia; MI = Myocardial infarction, ACS = Acute coronary syndrome. 1. Sabatine MS et al. , Circulation. 2018; pii: CIRCULATIONAHA. 118. 034309 2. Pedersen TR, et al. , Presented at ESC congress. 2017. Barcelona, Spain

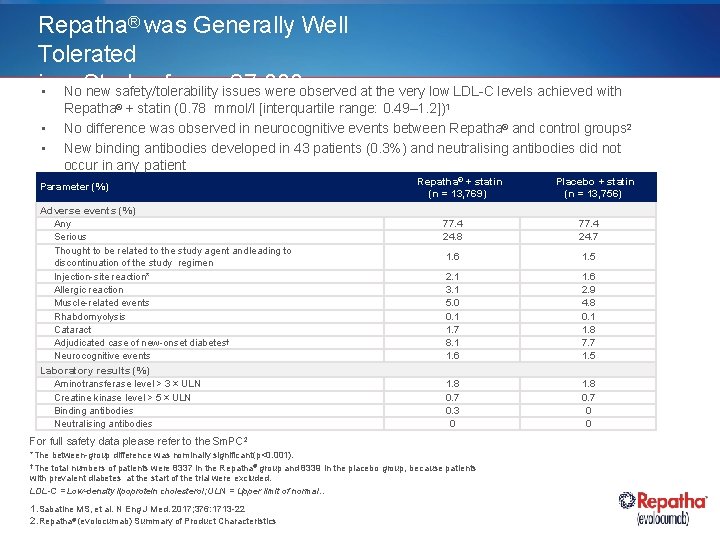
Repatha® was Generally Well Tolerated in of overissues 27, 000 • a No Study new safety/tolerability were observed at the very low LDL-C levels achieved with Repatha 2 + statin (0. 78 mmol/l [interquartile range: 0. 49– 1. 2]) Patients ® • • 1 No difference was observed in neurocognitive events between Repatha® and control groups 2 New binding antibodies developed in 43 patients (0. 3%) and neutralising antibodies did not occur in any patient Parameter (%) Adverse events (%) Any Serious Thought to be related to the study agent and leading to discontinuation of the study regimen Injection-site reaction* Allergic reaction Muscle-related events Rhabdomyolysis Cataract Adjudicated case of new-onset diabetes† Neurocognitive events Laboratory results (%) Aminotransferase level > 3 × ULN Creatine kinase level > 5 × ULN Binding antibodies Neutralising antibodies Repatha® + statin (n = 13, 769) 77. 4 24. 8 77. 4 24. 7 1. 6 1. 5 2. 1 3. 1 5. 0 0. 1 1. 7 8. 1 1. 6 2. 9 4. 8 0. 1 1. 8 7. 7 1. 5 1. 8 0. 7 0. 3 0 1. 8 0. 7 0 0 For full safety data please refer to the Sm. PC 2 *The between-group difference was nominally significant (p<0. 001). †The total numbers of patients were 8337 in the Repatha® group and 8339 in the placebo group, because patients with prevalent diabetes at the start of the trial were excluded. LDL-C = Low-density lipoprotein cholesterol; ULN = Upper limit of normal. . 1. Sabatine MS, et al. N Eng J Med. 2017; 376: 1713 -22 2. Repatha® (evolocumab) Summary of Product Characteristics Placebo + statin (n = 13, 756)

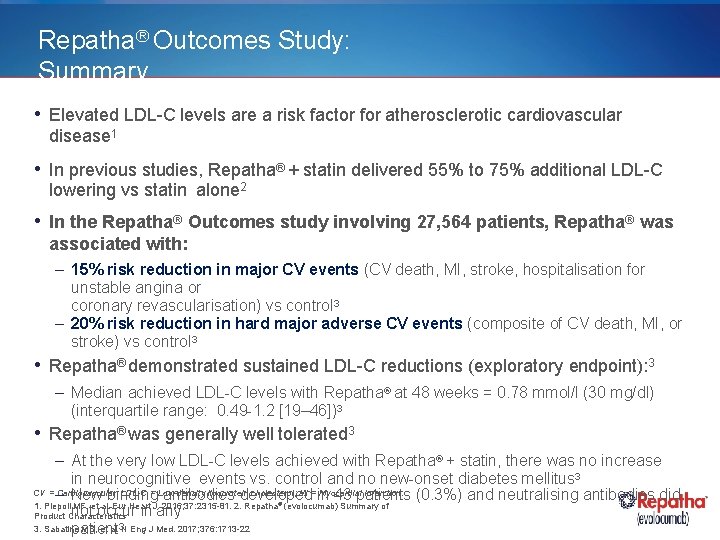
Repatha® Outcomes Study: Summary • Elevated LDL-C levels are a risk factor for atherosclerotic cardiovascular disease 1 • In previous studies, Repatha® + statin delivered 55% to 75% additional LDL-C lowering vs statin alone 2 • In the Repatha® Outcomes study involving 27, 564 patients, Repatha® was associated with: – 15% risk reduction in major CV events (CV death, MI, stroke, hospitalisation for unstable angina or coronary revascularisation) vs control 3 – 20% risk reduction in hard major adverse CV events (composite of CV death, MI, or stroke) vs control 3 • Repatha® demonstrated sustained LDL-C reductions (exploratory endpoint): 3 – Median achieved LDL-C levels with Repatha® at 48 weeks = 0. 78 mmol/l (30 mg/dl) (interquartile range: 0. 49 -1. 2 [19– 46])3 • Repatha® was generally well tolerated 3 – At the very low LDL-C levels achieved with Repatha® + statin, there was no increase in neurocognitive events vs. control and no new-onset diabetes mellitus 3 CV = – Cardiovascular; LDL-C = Low-density lipoprotein cholesterol; MI = in Myocardial infarction. (0. 3%) and neutralising antibodies did New binding antibodies developed 43 patients 1. Piepoli MF, et al. Eur Heart J. 2016; 37: 2315 -81. 2. Repatha (evolocumab) Summary of not occur in any Product Characteristics 3. Sabatine MS, et al. 3 N Eng J Med. 2017; 376: 1713 -22 patient ®

Repatha® (evolocumab) Brief Prescribing Information Repatha ® (evolocumab) Brief Prescribing Information Please refer to the Summary of Product Characteristics (Sm. PC) before prescribing Repatha. Pharmaceutical Form: Pre-filled pen (Sure. Click®) containing 140 mg of evolocumab in 1 m. L solution for injection. Indication: Hypercholesterolaemia and mixed dyslipidaemia: Repatha is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non‑familial) or mixed dyslipidaemia, as an adjunct to diet: in combination with a statin or statin with other lipid lowering therapies in patients unable to reach LDL‑C goals with the maximum tolerated dose of a statin or; alone or in combination with other lipidlowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. Homozygous familial hypercholesterolaemia: Repatha is indicated in adults and adolescents aged 12 years and over with homozygous familial hypercholesterolaemia in combination with other lipid-lowering therapies. Established atherosclerotic cardiovascular disease: Repatha is indicated in adults with established atherosclerotic cardiovascular disease (myocardial infarction, stroke or peripheral arterial disease) to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors: in combination with the maximum tolerated dose of a statin with or without other lipid-lowering therapies or; alone or in combination with other lipidlowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. For study results with respect to effects on LDL-C, cardiovascular events and populations studied see section 5. 1 of the Sm. PC. Dosage and Administration: Repatha is for subcutaneous injection into the abdomen, thigh or upper arm region. Repatha is intended for patient self-administration after proper training. Prior to initiating Repatha, secondary causes of hyperlipidaemia or mixed dyslipidaemia (e. g. , nephrotic syndrome, hypothyroidism) should be excluded. Primary hypercholesterolaemia and mixed dyslipidaemia in adults: The recommended dose of Repatha is either 140 mg every two weeks or 420 mg once monthly; both doses are clinically equivalent. The safety and efficacy of Repatha in children aged less than 18 years has not been established. Homozygous familial hypercholesterolaemia in adults and adolescents aged 12 years and over: The initial recommended dose is 420 mg once monthly. After 12 weeks of treatment, dose frequency can be up titrated to 420 mg once every 2 weeks if a clinically meaningful response is not achieved. Patients on apheresis may initiate treatment with 420 mg every two weeks to correspond with their apheresis schedule. The safety and efficacy of Repatha in children aged less than 12 years has not been established. Established atherosclerotic cardiovascular disease in adults: The recommended dose of Repatha is either 140 mg every two weeks or 420 mg once monthly; both doses are clinically equivalent. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Special Warnings and Precautions: Traceability: Clearly record the name and batch number of administered product to improve traceability of biological products. Hepatic impairment: In patients with moderate hepatic impairment, a reduction in total evolocumab exposure was observed that may lead to a reduced effect on LDL‑C reduction. Therefore, close monitoring may be warranted in these patients. Patients with severe hepatic impairment (Child-Pugh C) have not been studied. Repatha should be used with caution in patients with severe hepatic impairment. Dry natural rubber: The needle cover of the glass pre-filled pen (Sure. Click®) is made from dry natural rubber (a derivative of latex), which may cause severe allergic reactions. Interactions: No interaction studies have been performed. Fertility, pregnancy and lactation: There are no or limited amount of data from the use of Repatha in pregnant women. It is unknown whether evolocumab is excreted in human milk. A risk to breastfed newborns/infants cannot be excluded. No data on the effect of evolocumab on human fertility are available. Undesirable Effects: Adverse reactions reported in pivotal, controlled clinical studies and from spontaneous reporting: common (≥ 1/100 to < 1/10) influenza, nasopharyngitis, upper respiratory tract infection, hypersensitivity, rash, nausea, back pain, arthralgia, injection site reactions; rare (≥ 1/10, 000 to < 1/1, 000) angioedema. Please consult the Sm. PC for a full description of undesirable effects. Pharmaceutical Precautions: Store in a refrigerator (2 C – 8 C). Do not freeze. Store the pre-filled pen (Sure. Click®) in the original carton in order to protect from light. If removed from the refrigerator, Repatha may be stored at room temperature (up to 25°C) in the original carton and must be used within 1 month. Legal Category: POM. Presentation, Basic Costs and Marketing Authorisation Number: Repatha pre-filled pen (Sure. Click®) 140 mg/1 m. L: Pack of 2 pre-filled pens: £ 340. 20; EU/1/15/1016/003. Marketing Authorisation Holder: Amgen Europe B. V. Minervum 7061, 4817 ZK Breda, The Netherlands. Further information is available from Amgen Limited, 240 Cambridge Science This medicinal product is subject to additional monitoring. Adverse Park, Milton Road, Cambridge, CB 4 0 WD. Repatha is a registered trademark of Amgen Inc. events should be reported. Reporting forms and Date of PI preparation: April 2020 information can(Ref: UKIE-P-145 -0916 -038056(5)). be found at www. mhra. gov. uk/yellowcard. Adverse events should also be reported to Amgen Limited +44 be (0)reported. 1223 436441. Adverse events on should Reporting forms and information can be found at https: //yellowcard. mhra. gov. uk. Adverse events should also be reported to Amgen Limited on +44 (0) 1223 436441.
 Psck 9
Psck 9 Repatha opstart verpleegkundige
Repatha opstart verpleegkundige Clowering
Clowering Repatha refrigeration
Repatha refrigeration Niclas abrahamsson uppsala
Niclas abrahamsson uppsala Diabetes prevention program outcomes study
Diabetes prevention program outcomes study Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau