Research Process Preclinical research Clinical Trials Ethics of

















































- Slides: 76


목차 • Research Process – Preclinical research – Clinical Trials • Ethics of Clinical trial • IRB • Clinical Trial Center 2

Pre-clinical Research • Information on safety and efficacy • Pharmaceutical company, FDA, IRB • Data – acute toxicity, – Kinetics and metabolism of the drug – Organ sensitivity • Determine a starting dose 3

Drug discovery • Create compounds • Change the molecular structure • Structure/function/activity/gene sequencing • Computation and computer modeling 4

Pre-clinical studies • Determine the basic physical, chemical and biological characteristics – stability, formulation, bioavailability • Safety studies – – Genotoxicity, singe & multiple(repeated) dose toxicology, local tolerance, teratology or reproductive S. 5

Single dose toxicity studies • • • Acute toxicity studies two non-human mammalian species Dose escalation studies During a period of 24 hours or less 14 -day non-treatment observation Choosing the doses for repeated dose studies 6

Repeated dose studies • • • Sub-chronic & chronic toxicology S. Period of time consistent two non-human mammalian species Equal or exceed the length of the clinical trials The longest chronic toxicity study : nine to twelve months 7

Safety Pharmacology studies • Effect of the drug on vital functions – Respiratory, CNS, CVS in animal • Separately or with toxicology S. • What the drug does to the body at pharmacological doses. 8

Pharmacokinetic studies • Identify absorption, distribution, metabolism, excretion of the test substance 9

Genotoxicity studies • If mutations or chromosomal damage • Positive findings additional testing • Indication for carcinogenicity testing 10

Carcinogenicity studies • Continuously for six months or longer or intermittently for periods that equal six or more months of continuous use • Not required for drugs: – Anti-cancer drugs, anesthetics • In rats and mice 11

Reproductive toxicity studies • Segment I ; – studies of fertility and reproductive performance – one non-human species, rat • Segment II ; – effect on the fetus(teratology) – two non-human species, rat and rabbit • Segment III ; – peri and postnatal portion assessing the effect on the unborn or litters – two non-human species 12

Data collected in animal studies • Daily clinical observation with palpation, body weight, food consumption measurements • Hematology, clinical chemistry and urinalysis • Electrocardiograms • Ophthalmic examinations • Neurobehavioral testing 13

Regulatory guidelines • GLP • IACUC(Institutional Animal Care and Use Committee) • Animal Welfare Act 14

IND Application • Animal pharmacology and toxicology studies; pre-clinical data • Any previous experience in humans from non. U. S. studies • Chemistry, manufacturing and control information • Protocol and investigator information • Assurance of IRB • General develoment plan for the drug • Responsibilities of CRO 15

IND Application • 30 days • Updated on an annual basis • Amendents at any time 16

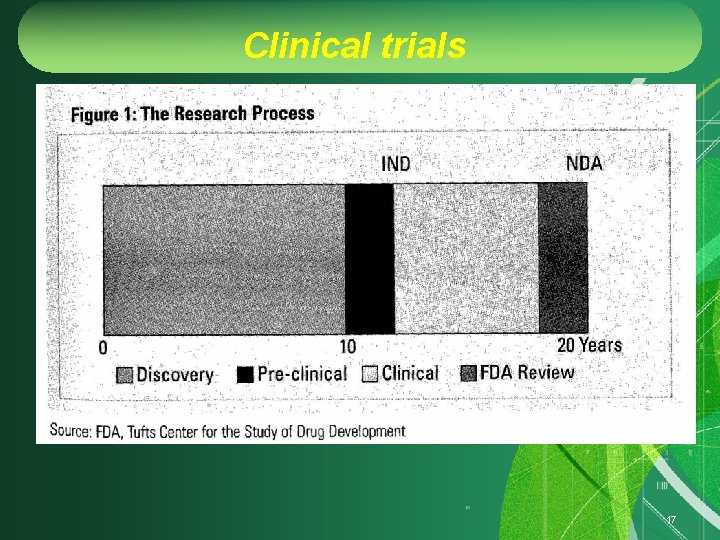
Clinical trials 17

Phase I Clinical Trials • • Safety studies Small number; 20 -100 Healthy volunteers (no anticancer drug) Determine the metabolic and pharmacologic action • Assess the adverse effects associated with different doses(max. dose) • Get an indication of efficacy 18

Phase II Clinical Trials • • A few hundred subjects in target disease. Double blind, well-controlled, Using a placebo or comparator drug Demonstrates efficacy for the proposed indication within the safe dose range • Assess the short-term adverse effects and risks(safety) • Dose range finding – Min. & max. effective dose, PK/PD data 19

Phase III Clinical Trials • Demonstrate the safety and efficacy • Assess the risk/benefit relationship for the intended use • Provide adequate data for the product package insert • Thousands of patients, Several years • Multicenter studies • Adequate & well controlled(primary E. ) 20

NDA • • Submits the NDA to the FDA The end of phase III (safety & efficacy) Regulation(protocol, CRF, inset paper. . ) Approval letter from the FDA 21

Phase IIIb Clinical Trials • Initiated and conducted while the NDA is pending approval • Gather additional safety data or information on additional indications • Assess its use in special patient populations such as geriatric patients 22

Phase IV Clinical Trials • Done after approval of the NDA • Determine additional information about the safety or efficacy profile • Required as a condition of approval • Long-term safety studies required • Comparison with other products • Designed to familiarize physicians 23

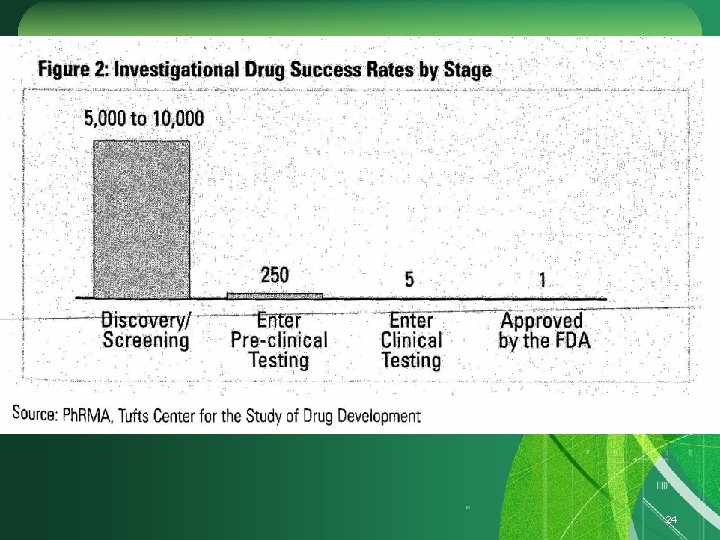
24

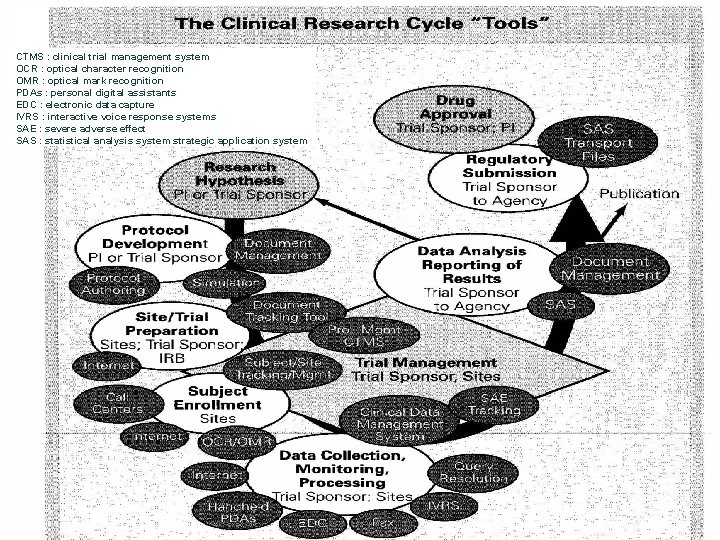
CTMS : clinical trial management system OCR : optical character recognition OMR : optical mark recognition PDAs : personal digital assistants EDC : electronic data capture IVRS : interactive voice response systems SAE : severe adverse effect SAS : statistical analysis system strategic application system 25


27












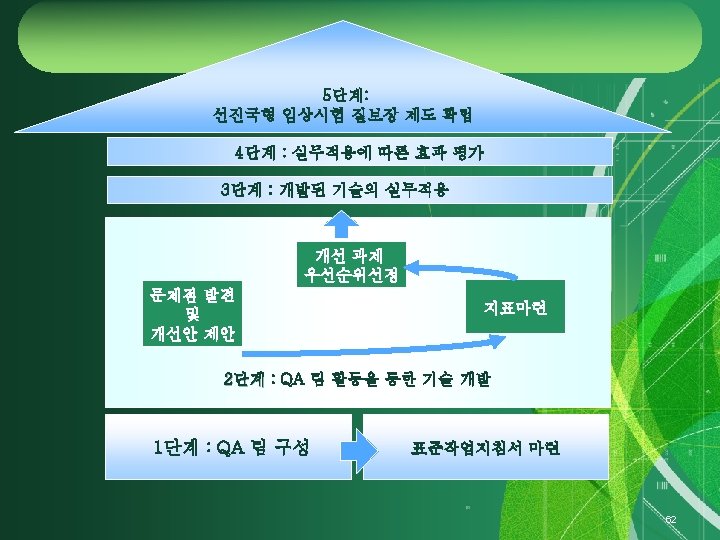
IRB 39


Responsibilities of Clinical Site Staff • Ensuring that written approval from the IRB in accordance with ICH GCP before the start of the study, • Knowing when the IRB is required to be informed about any changes and safety aspects while the study is being conducted, • Informing the IRB about safety issues during the study according to local requirements or as demanded by the IRB and about completion of the clinical study. 41

Responsibilities of Clinical Site Staff • Provide detailed information verbally as well as in writing about the study to the study subjects; • Try to ensure that each subject understands the procedures and the requirements of the study, especially the actions you expect her/him to take; • Take care that the subject realizes the voluntariness of her/his participation in the trial • Obtain written consent from each study subject before performing any study-specific activity. 42








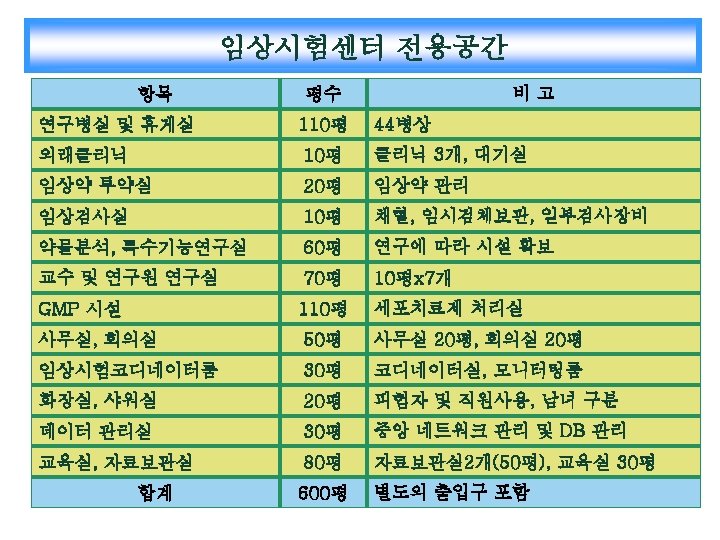
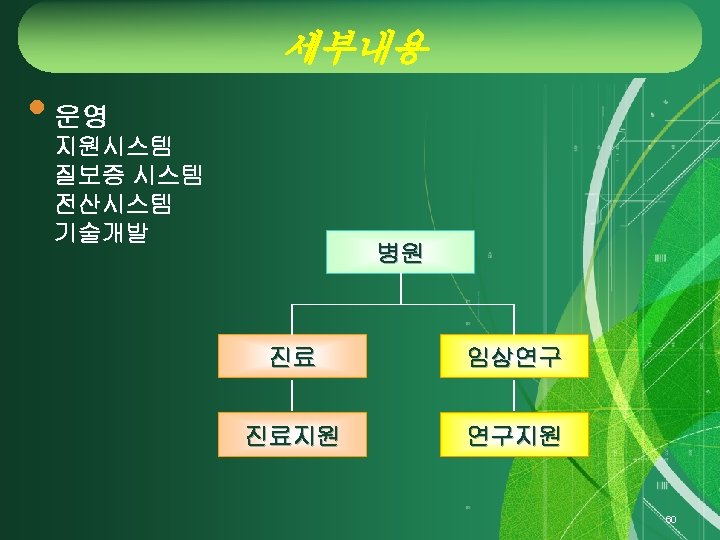
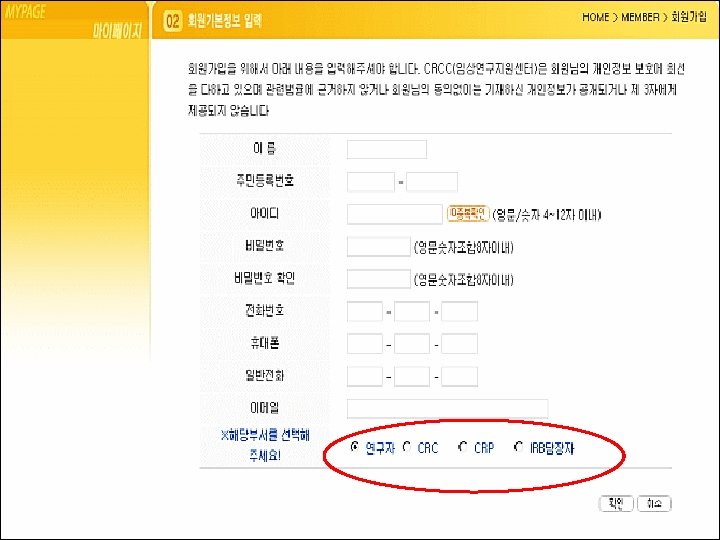
Clinical Trial Center 50




강남성모병원 ISO 9001 인증 2005. 9. 27 의료 분야의 품질경영을 선도 QM(품질경영) Quality Management 품질기획 Planning 품질관리 Control 품질보증 Assurance 품질개선 Improvement 54










메인 http: //cmccrcc. catholic. ac. kr 64


66


68

69

70

71

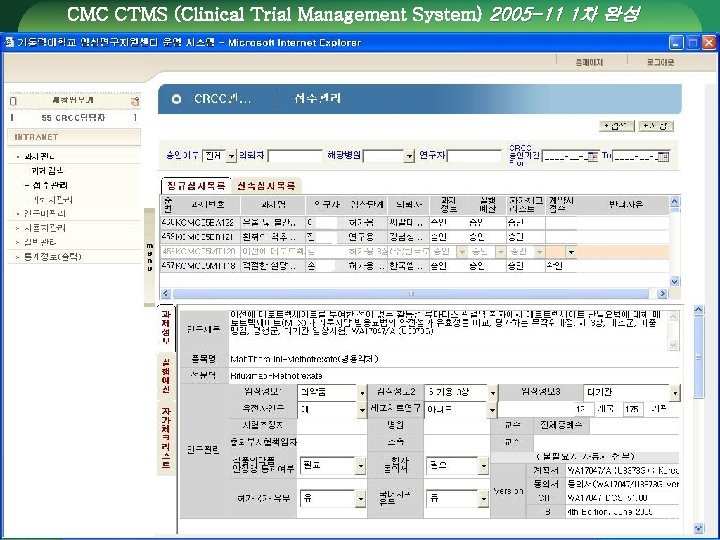
CMC CTMS (Clinical Trial Management System) 2005 -11 1차 완성 72

73

74


Thanks for your attention !! 76