Preclinical Services at NIAIDNIH for Drug Development Zuoyu








- Slides: 11

Preclinical Services at NIAID/NIH for Drug Development Zuoyu Xu, Ph. D. Program Officer National Institute of Allergy and Infectious Diseases NIH/DHHS April 15, 2015

The National Institute of Allergy and Infectious Diseases (NIAID) Conducts and supports basic and applied research to better understand, treat, and ultimately prevent infectious, immunologic, and allergic diseases

The Division of Microbiology and Infectious Diseases (DMID) Supports basic research through applied research to control and prevent diseases caused by virtually all human infectious agents except HIV (managed by the Division of AIDS) Photo credit: NIAID RML

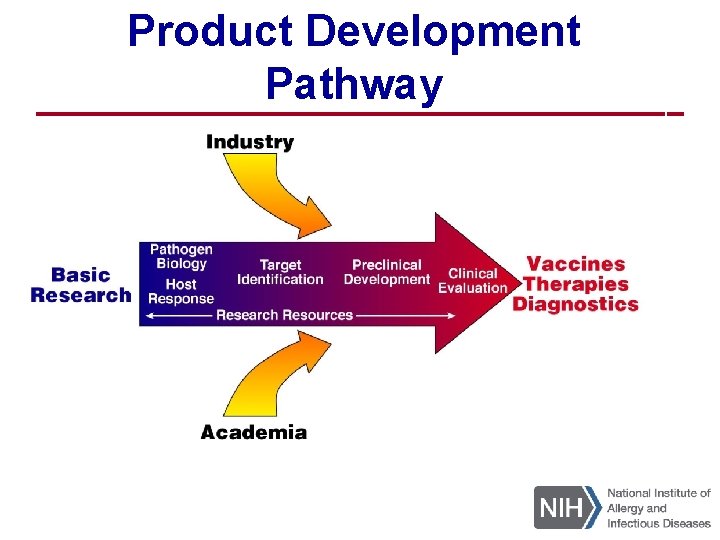
Product Development Pathway

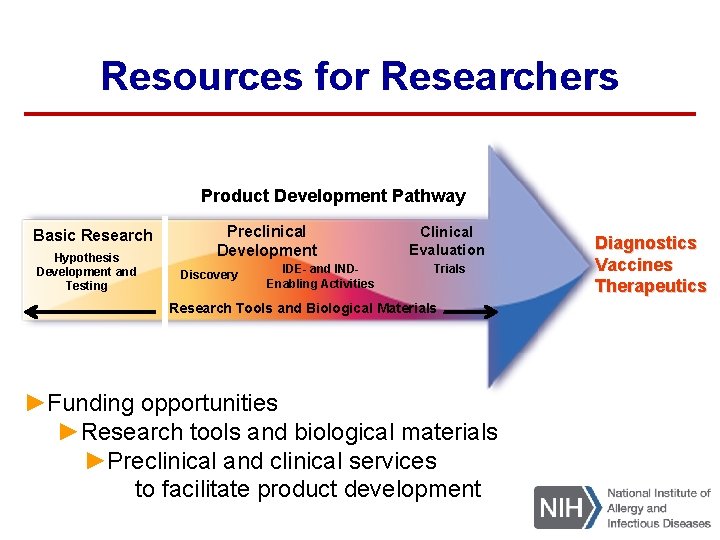
Resources for Researchers Product Development Pathway Basic Research Hypothesis Development and Testing Preclinical Development Discovery Clinical Evaluation IDE- and INDEnabling Activities Trials Research Tools and Biological Materials Research Tools and Technologies ►Funding opportunities ►Research tools and biological materials ►Preclinical and clinical services to facilitate product development Diagnostics Vaccines Therapeutics

Preclinical Services – Goal Help researchers move promising discoveries along the product development pathway

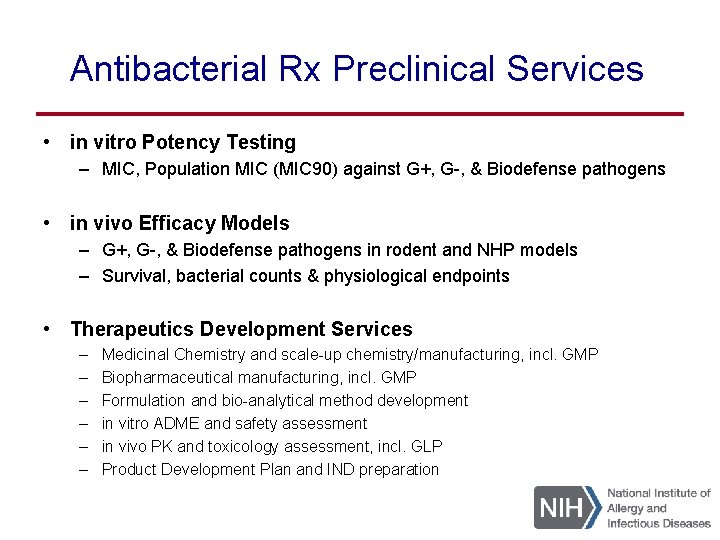
Antibacterial Rx Preclinical Services • in vitro Potency Testing – MIC, Population MIC (MIC 90) against G+, G-, & Biodefense pathogens • in vivo Efficacy Models – G+, G-, & Biodefense pathogens in rodent and NHP models – Survival, bacterial counts & physiological endpoints • Therapeutics Development Services – – – Medicinal Chemistry and scale-up chemistry/manufacturing, incl. GMP Biopharmaceutical manufacturing, incl. GMP Formulation and bio-analytical method development in vitro ADME and safety assessment in vivo PK and toxicology assessment, incl. GLP Product Development Plan and IND preparation

Eligibility Criteria – Who Can use the Services? • Investigators in academia, not-for-profit organizations, industry, and government • National/international institutions • NIH funding not required • Simplified Request Process – available yearround

Requirements & Assurances • Requirements of Users may include: – Shipping and handling charges for samples – Acknowledging the contribution of NIAID contract support in publications and presentations – Providing citations for presentations related to work supported by preclinical services – Reporting achievements to NIAID annually • Assurances Provided to Users: – – Confidentiality Materials Transfer Agreement (MTA) Non-Clinical Evaluation Agreement (NCEA) Clinical Trial Agreement (CTA)

Preclinical Services – How to Access • Available through application - contact Program Officer – Resources are limited – Gap filling – To provide critical information needed to move a product forward – Not intended as the sole source of development – Preliminary data required to proceed through each stage of development

 Preclinical stage
Preclinical stage Pathway preclinical services
Pathway preclinical services Preclinical research services
Preclinical research services Preclinical research services
Preclinical research services Niaid preclinical services
Niaid preclinical services Role of computer in preclinical development
Role of computer in preclinical development An example of crude drug adulterated with exhausted drug
An example of crude drug adulterated with exhausted drug Preclinical studies
Preclinical studies Preclinical studies
Preclinical studies Define ibogaine
Define ibogaine Preclinical studies
Preclinical studies Career opportunities in biotechnology and drug development
Career opportunities in biotechnology and drug development