PreClinical Safety Studies with the LUTONIX 035 Renu




- Slides: 20

Pre-Clinical Safety Studies with the LUTONIX® 035 Renu Virmani MD CVPath Institute, Gaithersburg, Maryland www. cvpath. org

Speaker's name: Renu Virmani, MD I have the following potential conflicts of interest to report: Ø Consultant: 480 Biomedical, Abbott Vascular, Medtronic, and W. L. Gore. Ø Employment in industry: No Ø Honorarium: 480 Biomedical, Abbott Vascular, Boston Scientific, Cordis J&J, Lutonix, Medtronic, Merck, Terumo Corporation, and W. L. Gore. Ø Institutional grant/research support: 480 Biomedical, Abbott Vascular, Atrium, Bio. Sensors International, Biotronik, Boston Scientific, Cordis J&J, GSK, Kona, Medtronic, Micro. Port Medical, Celo. Nova, Orbus. Neich Medical, Re. Core, SINO Medical Technology, Terumo Corporation, and W. L. Gore. Ø Owner of a healthcare company: No Ø Stockholder of a healthcare company: No

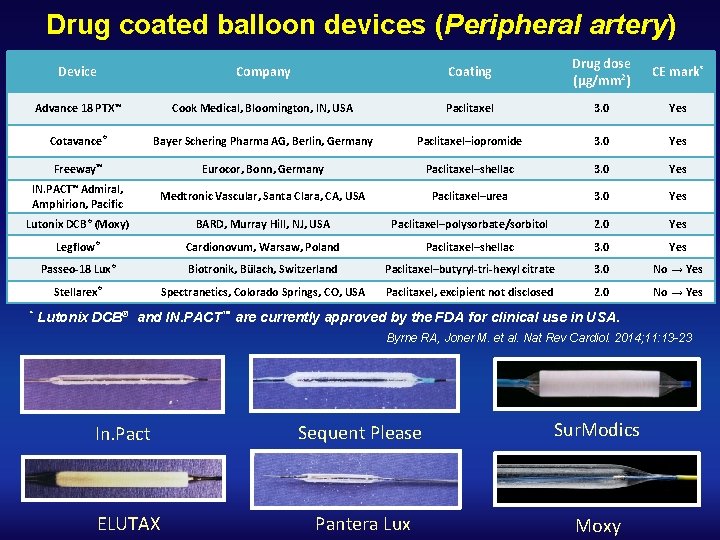
Drug coated balloon devices (Peripheral artery) Device Company Coating Drug dose (µg/mm 2) CE mark* Advance 18 PTX™ Cook Medical, Bloomington, IN, USA Paclitaxel 3. 0 Yes Cotavance® Bayer Schering Pharma AG, Berlin, Germany Paclitaxel–iopromide 3. 0 Yes Freeway™ Eurocor, Bonn, Germany Paclitaxel–shellac 3. 0 Yes IN. PACT™ Admiral, Amphirion, Pacific Medtronic Vascular, Santa Clara, CA, USA Paclitaxel–urea 3. 0 Yes Lutonix DCB ® (Moxy) BARD, Murray Hill, NJ, USA Paclitaxel–polysorbate/sorbitol 2. 0 Yes Legflow® Cardionovum, Warsaw, Poland Paclitaxel–shellac 3. 0 Yes Passeo-18 Lux® Biotronik, Bülach, Switzerland Paclitaxel–butyryl-tri-hexyl citrate 3. 0 No → Yes Stellarex® Spectranetics, Colorado Springs, CO, USA Paclitaxel, excipient not disclosed 2. 0 No → Yes * Lutonix DCB® and IN. PACT™ are currently approved by the FDA for clinical use in USA. Byrne RA, Joner M. et al. Nat Rev Cardiol. 2014; 11: 13 -23 In. Pact ELUTAX Sequent Please Sur. Modics Pantera Lux Moxy

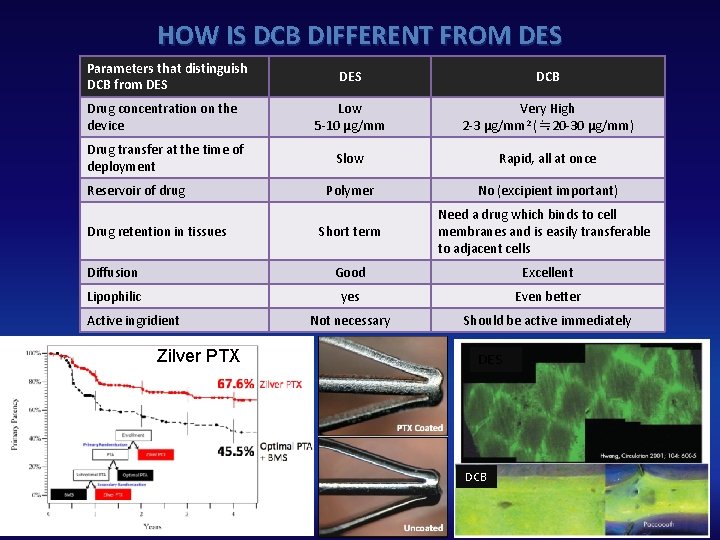
HOW IS DCB DIFFERENT FROM DES Parameters that distinguish DCB from DES DCB Drug concentration on the device Low 5‐ 10 μg/mm Very High 2‐ 3 μg/mm 2 (≒ 20‐ 30 μg/mm) Drug transfer at the time of deployment Slow Rapid, all at once Polymer No (excipient important) Short term Need a drug which binds to cell membranes and is easily transferable to adjacent cells Diffusion Good Excellent Lipophilic yes Even better Not necessary Should be active immediately Reservoir of drug Drug retention in tissues Active ingridient Zilver PTX DES DCB

Requirements For DCB • Must deliver large quantities of the drug within seconds • Must distribute within the media in the first few days • Therapeutic drug levels must be maintained for at least several weeks • Must allow rapid healing as compared to DES • No need for long‐term anticoagulation • Light microscopy must show biologic effects at 28‐days at least

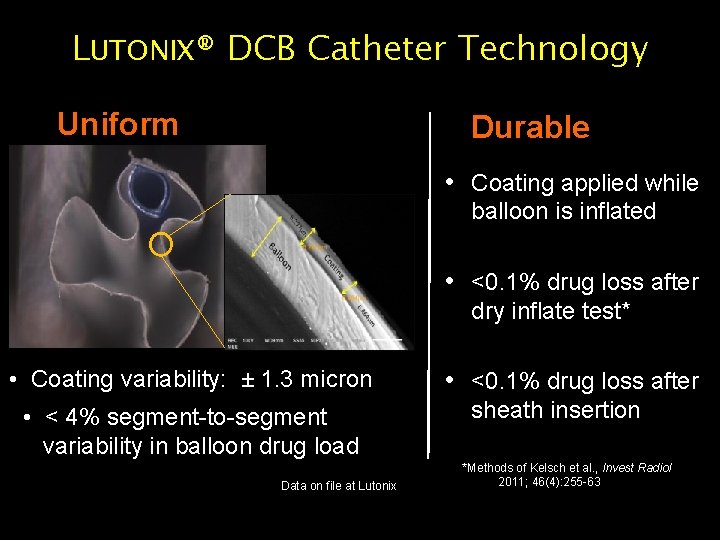
LUTONIX® DCB Catheter Technology Uniform Durable • Coating applied while balloon is inflated • <0. 1% drug loss after dry inflate test* • Coating variability: ± 1. 3 micron • < 4% segment-to-segment variability in balloon drug load Data on file at Lutonix CAUTION: Investigational Device – Limited by Federal (USA) Law to Investigational Use • <0. 1% drug loss after sheath insertion *Methods of Kelsch et al. , Invest Radiol 2011; 46(4): 255 -63

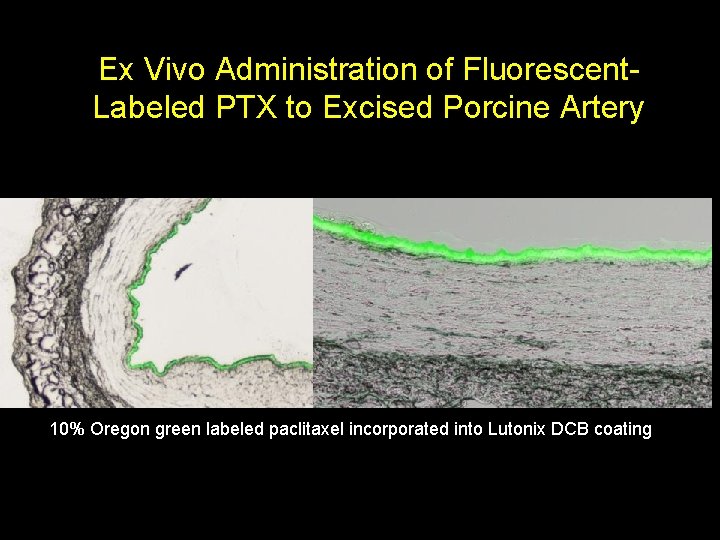
Ex Vivo Administration of Fluorescent. Labeled PTX to Excised Porcine Artery 10% Oregon green labeled paclitaxel incorporated into Lutonix DCB coating CAUTION: Investigational Device – Limited by Federal (USA) Law to Investigational Use

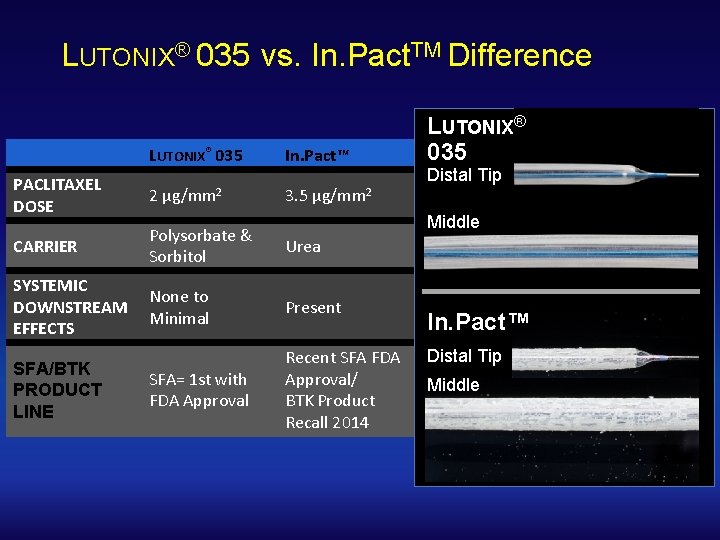
LUTONIX® 035 vs. In. Pact. TM Difference LUTONIX® 035 In. Pact™ PACLITAXEL DOSE 2 CARRIER Polysorbate & Sorbitol Urea SYSTEMIC DOWNSTREAM EFFECTS None to Minimal Present SFA= 1 st with FDA Approval Recent SFA FDA Approval/ BTK Product Recall 2014 SFA/BTK PRODUCT LINE μg/mm 2 3. 5 μg/mm 2 LUTONIX® 035 Distal Tip Middle In. Pact™ Distal Tip Middle

Coating Integrity is Variable PACLITAXEL LUTONIX® 035 PACLITAXEL IN COATING AFTER AQUEOUS EXPOSURE In. Pact™

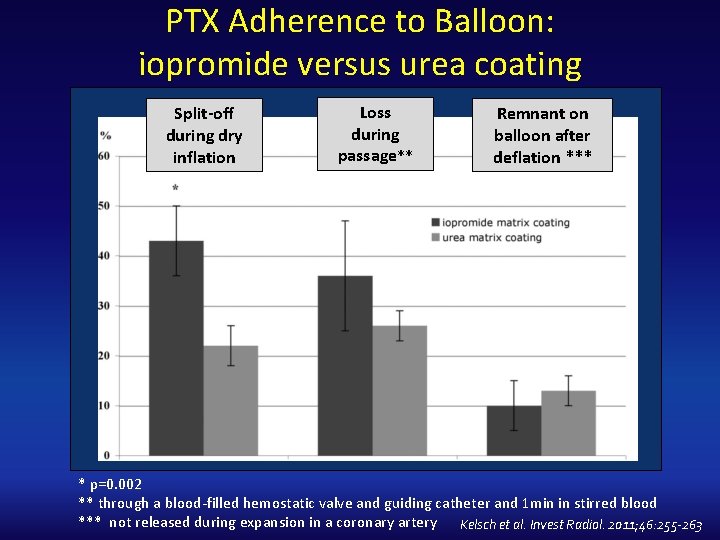
PTX Adherence to Balloon: iopromide versus urea coating Split-off during dry inflation Loss during passage** Remnant on balloon after deflation *** * p=0. 002 ** through a blood‐filled hemostatic valve and guiding catheter and 1 min in stirred blood *** not released during expansion in a coronary artery Kelsch et al. Invest Radiol. 2011; 46: 255 -263

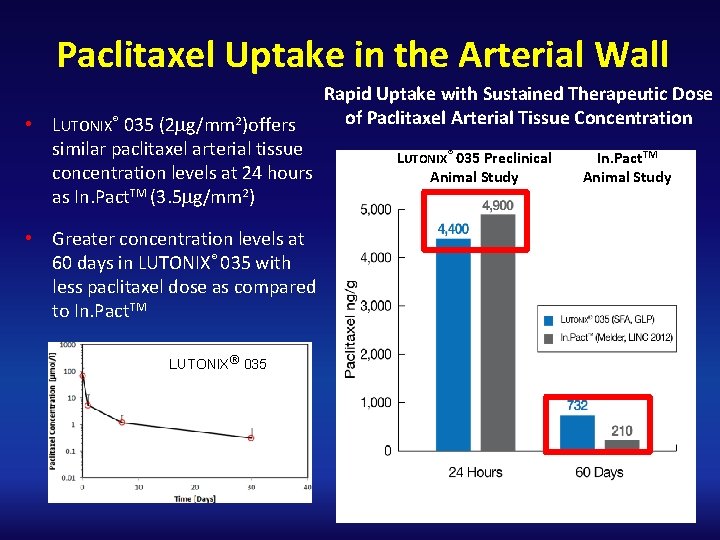
Paclitaxel Uptake in the Arterial Wall • LUTONIX® 035 (2 mg/mm 2)offers similar paclitaxel arterial tissue concentration levels at 24 hours as In. Pact. TM (3. 5 mg/mm 2) • Greater concentration levels at 60 days in LUTONIX® 035 with less paclitaxel dose as compared to In. Pact. TM LUTONIX® 035 Rapid Uptake with Sustained Therapeutic Dose of Paclitaxel Arterial Tissue Concentration LUTONIX® 035 Preclinical Animal Study In. Pact. TM Animal Study

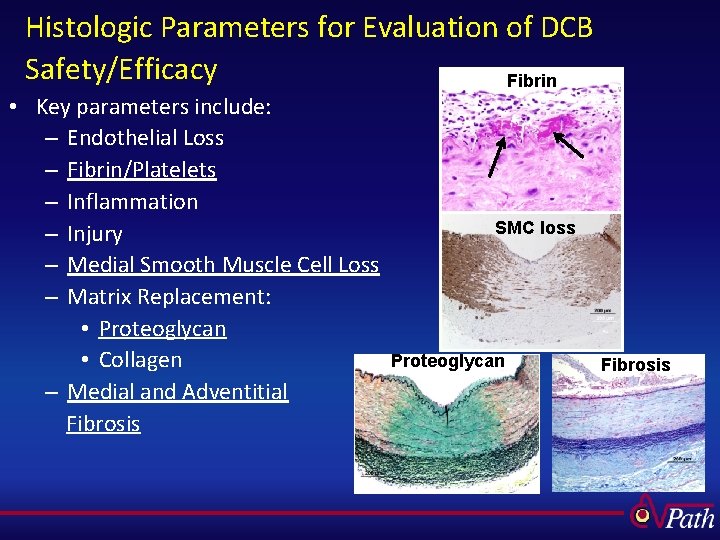
Histologic Parameters for Evaluation of DCB Safety/Efficacy Fibrin • Key parameters include: – Endothelial Loss – Fibrin/Platelets – Inflammation – Injury – Medial Smooth Muscle Cell Loss – Matrix Replacement: • Proteoglycan • Collagen – Medial and Adventitial Fibrosis SMC loss Proteoglycan Fibrosis

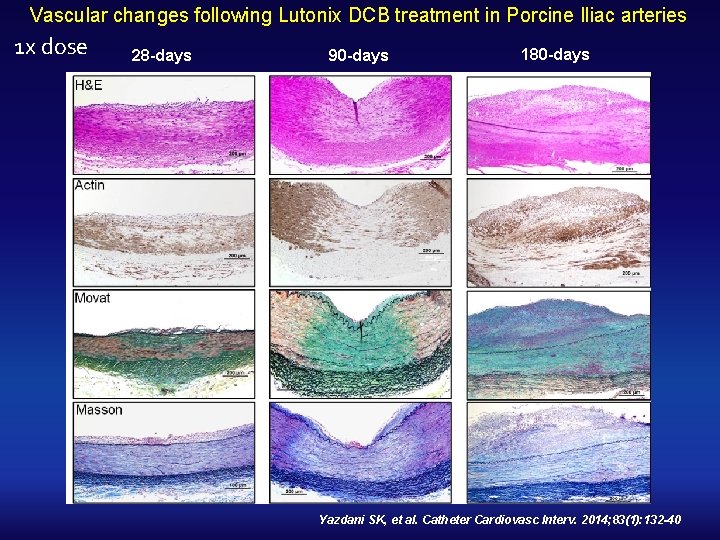
Vascular changes following Lutonix DCB treatment in Porcine Iliac arteries 1 x dose 28 -days 90 -days 180 -days Yazdani SK, et al. Catheter Cardiovasc Interv. 2014; 83(1): 132 -40

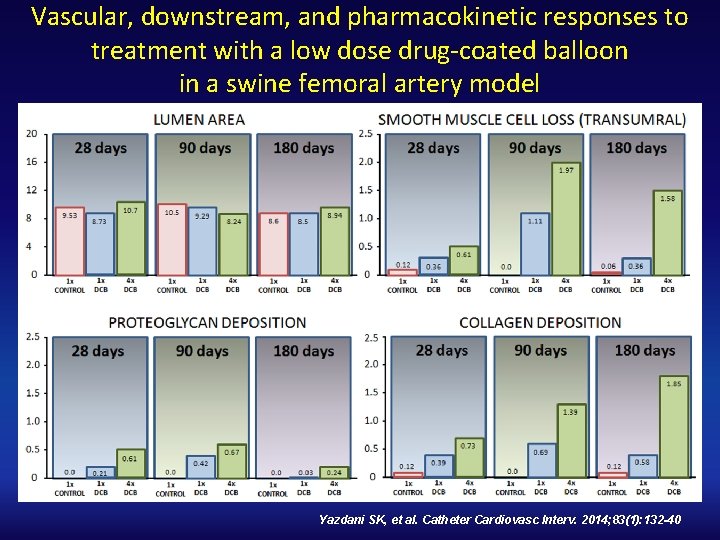
Vascular, downstream, and pharmacokinetic responses to treatment with a low dose drug‐coated balloon in a swine femoral artery model Yazdani SK, et al. Catheter Cardiovasc Interv. 2014; 83(1): 132 -40

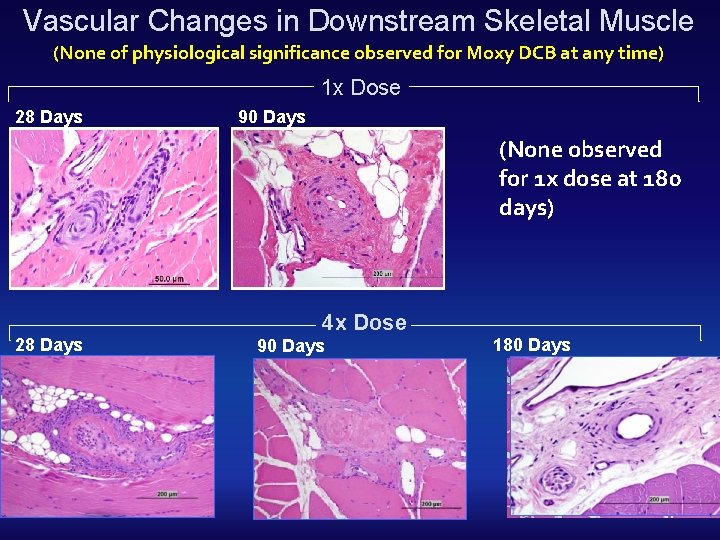
Vascular Changes in Downstream Skeletal Muscle (None of physiological significance observed for Moxy DCB at any time) 1 x Dose 28 Days 90 Days (None observed for 1 x dose at 180 days) 28 Days 4 x Dose 90 Days 180 Days

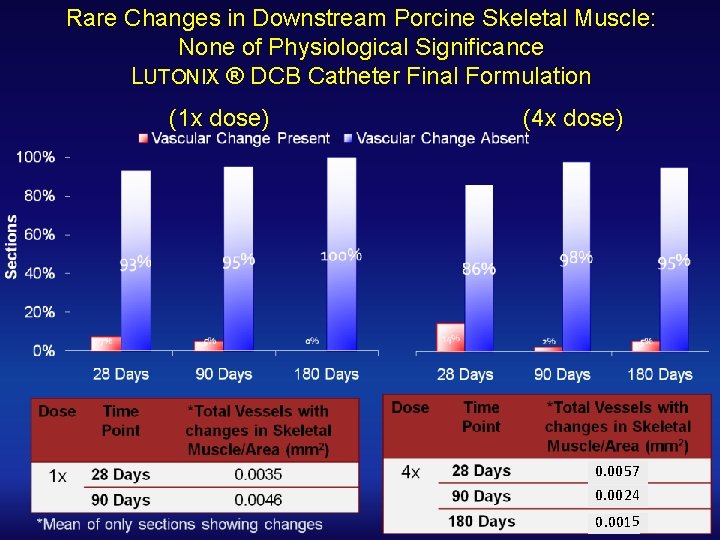
Rare Changes in Downstream Porcine Skeletal Muscle: None of Physiological Significance LUTONIX ® DCB Catheter Final Formulation (1 x dose) (4 x dose) 0. 0057 0. 0024 0. 0015

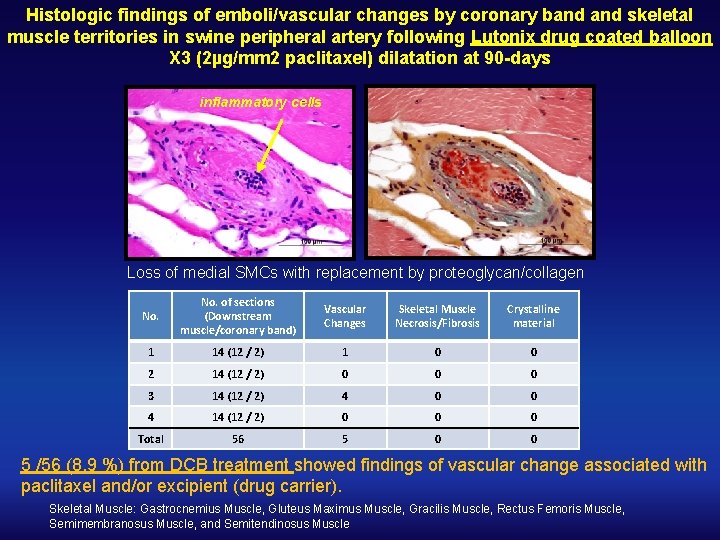
Histologic findings of emboli/vascular changes by coronary band skeletal muscle territories in swine peripheral artery following Lutonix drug coated balloon X 3 (2µg/mm 2 paclitaxel) dilatation at 90 -days inflammatory cells Loss of medial SMCs with replacement by proteoglycan/collagen No. of sections (Downstream muscle/coronary band) Vascular Changes Skeletal Muscle Necrosis/Fibrosis Crystalline material 1 14 (12 / 2) 1 0 0 2 14 (12 / 2) 0 0 0 3 14 (12 / 2) 4 0 0 4 14 (12 / 2) 0 0 0 Total 56 5 0 0 5 /56 (8. 9 %) from DCB treatment showed findings of vascular change associated with paclitaxel and/or excipient (drug carrier). Skeletal Muscle: Gastrocnemius Muscle, Gluteus Maximus Muscle, Gracilis Muscle, Rectus Femoris Muscle, Semimembranosus Muscle, and Semitendinosus Muscle

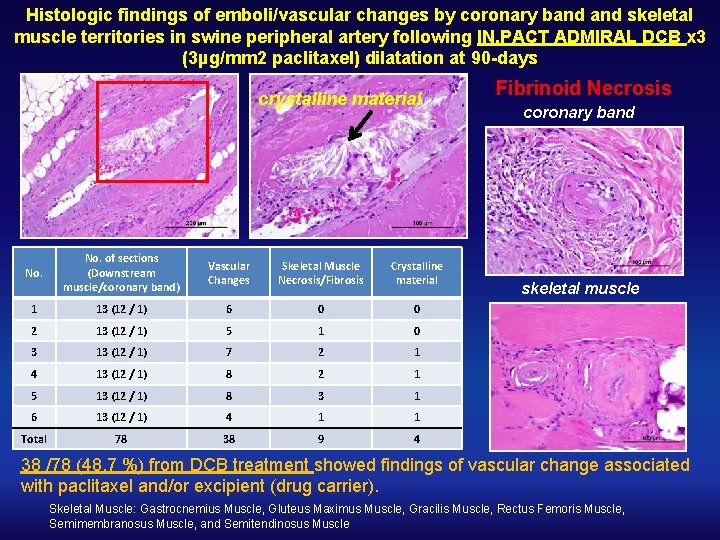
Histologic findings of emboli/vascular changes by coronary band skeletal muscle territories in swine peripheral artery following IN. PACT ADMIRAL DCB x 3 (3µg/mm 2 paclitaxel) dilatation at 90 -days crystalline material No. of sections (Downstream muscle/coronary band) Vascular Changes Skeletal Muscle Necrosis/Fibrosis Crystalline material 1 13 (12 / 1) 6 0 0 2 13 (12 / 1) 5 1 0 3 13 (12 / 1) 7 2 1 4 13 (12 / 1) 8 2 1 5 13 (12 / 1) 8 3 1 6 13 (12 / 1) 4 1 1 Total 78 38 9 4 Fibrinoid Necrosis coronary band skeletal muscle 38 /78 (48. 7 %) from DCB treatment showed findings of vascular change associated with paclitaxel and/or excipient (drug carrier). Skeletal Muscle: Gastrocnemius Muscle, Gluteus Maximus Muscle, Gracilis Muscle, Rectus Femoris Muscle, Semimembranosus Muscle, and Semitendinosus Muscle

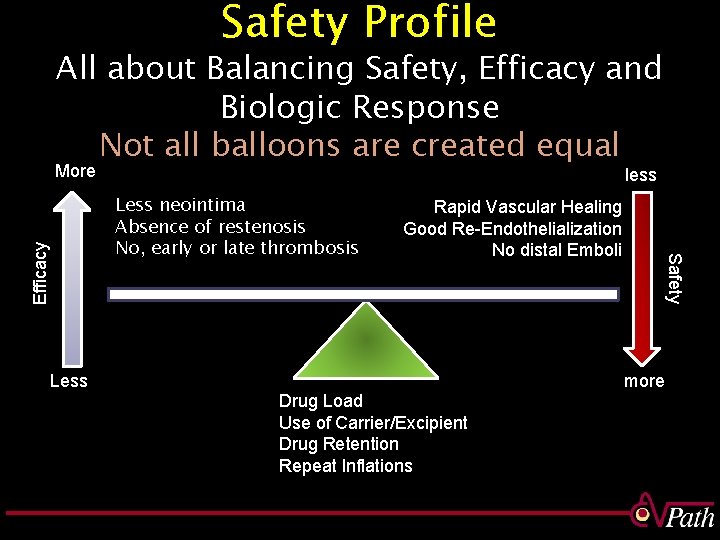
Safety Profile All about Balancing Safety, Efficacy and Biologic Response Not all balloons are created equal More less Efficacy Less Rapid Vascular Healing Good Re-Endothelialization No distal Emboli Drug Load Use of Carrier/Excipient Drug Retention Repeat Inflations Safety Less neointima Absence of restenosis No, early or late thrombosis more

Acknowledgments CVPath Institute Kazuyuki Yahagi, MD Oscar D. Sanchez, MD Tobias R. Koppara, MD Erica Pacheco, MS Robert Kutz, MS Russ Jones Ed Acampado, DVM Youhui Liang, MD Abebe Atiso, HT Jinky Beyer Hedwig Avallone, HT Lila Adams, HT Hengying Ouyang, MD Elena Ladich, MD Frank D Kolodgie, Ph. D Michael Joner, MD Washington DC
 Preclinical studies
Preclinical studies Preclinical studies
Preclinical studies Define ibogaine
Define ibogaine Preclinical studies
Preclinical studies Nom 035
Nom 035 Disability resource center slcc
Disability resource center slcc Afoe nom 035
Afoe nom 035 55088-035
55088-035 Renu ramnarayanan
Renu ramnarayanan Renu 28 asea
Renu 28 asea Asea renu 28 ingredients
Asea renu 28 ingredients Renu is recognised
Renu is recognised Renu 28 for burns
Renu 28 for burns Quoted and reported speech
Quoted and reported speech Renu 28 test
Renu 28 test Paradigm shift from women studies to gender studies
Paradigm shift from women studies to gender studies Preclinical research services
Preclinical research services Which information system used in preclinical development
Which information system used in preclinical development Preclinical research services
Preclinical research services What is preclinical
What is preclinical Niaid preclinical services
Niaid preclinical services